Phoenix™
Trusted PK/PD analysis for preclinical and clinical insights
New 8.6 version is now available
Download
Phoenix 8.6 version is now available
All-in-one pharmacokinetic and pharmacodynamic decision support
Phoenix™ delivers comprehensive tools for PK/PD modeling, empowering preclinical scientists and clinical pharmacologists to make critical drug development decisions like these with confidence, even for the most complex modalities.
Versatility for the PK/PD Scientist
Certara Phoenix bridges the gap between discovery and clinical phases by enabling robust analysis of pharmacokinetic (PK) and pharmacodynamic (PD) data in every application. From early preclinical evaluations to late stage regulatory submissions, Phoenix ensures precision, scalability, and compliance, supporting decision making across the drug development spectrum.
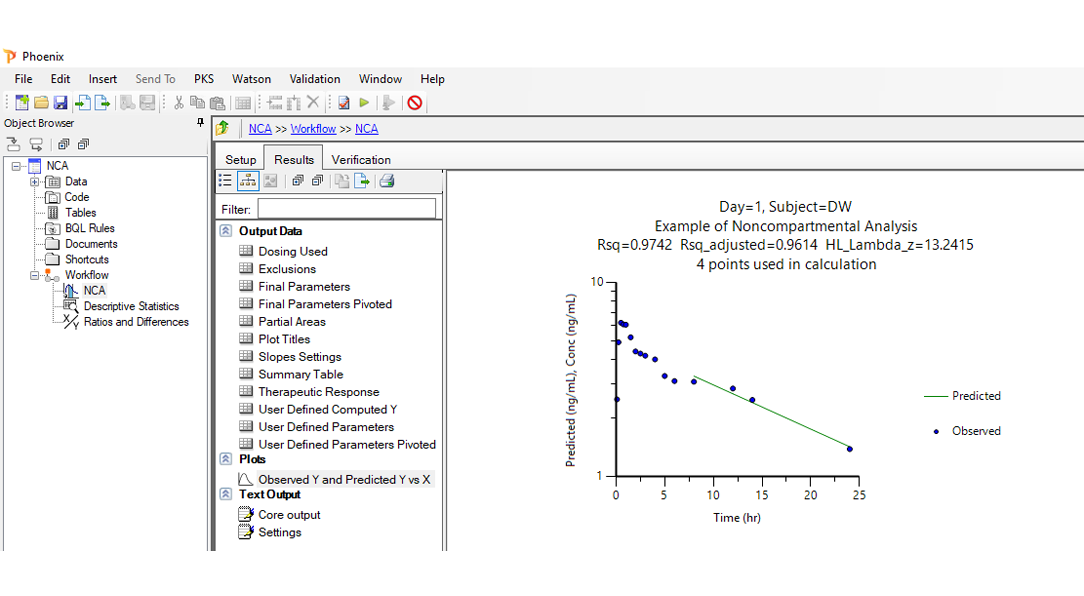
Noncompartmental analysis (NCA):
Precise NCA with flexible data handling, regulatory compliance, and intuitive visualization
Learn more
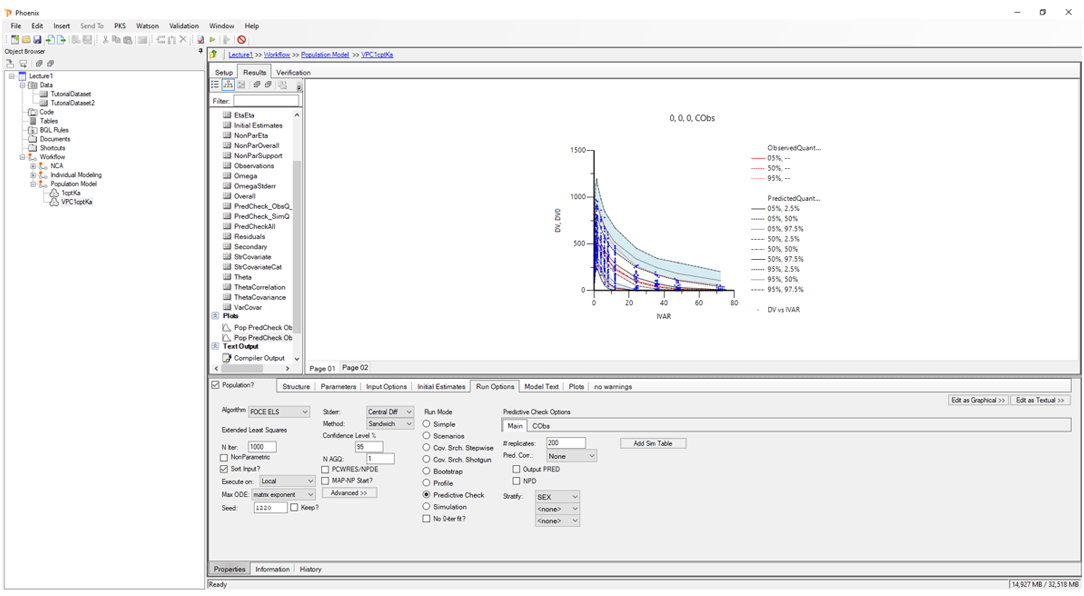
Population PK/PD:
Evaluate population-level data using NLME modeling with an intuitive interface.
Learn more
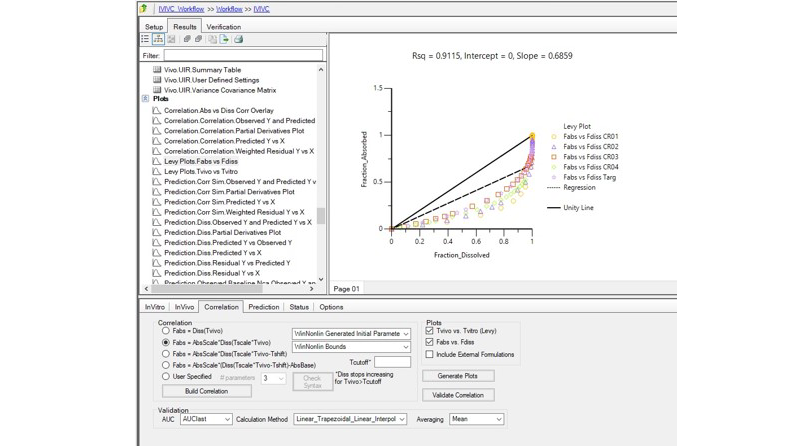
In Vitro-In Vivo Correlation (IVIVC):
Seamlessly correlate in vitro dissolution data with in vivo performance to optimize formulations.
Learn more
The industry standard in PK/PD analysis
Phoenix for every task
The Phoenix Platform has been adopted by 11 global regulatory agencies, including the US FDA
Enhance your knowledge in PK analysis. Access comprehensive insights from distinguished experts in pharmacokinetics and pharmacodynamics.
PK/PD resources
View all
With Phoenix, your data is secure
Certara holds ISO 27001 certification for Certara’s Information Security Management System (ISMS). We have implemented robust security controls, undergone rigorous risk assessments, and continuously strive for improvement. Phoenix ensures full compliance with global data protection standards, offering peace of mind for sensitive analysis.
Schedule a Phoenix demo
Ready to See Phoenix in Action? Transform your PK/PD analyses with a guided demo tailored to your needs. We’ll show you how Phoenix can bridge your journey across drug development.
We’re committed to delivering content that truly matters to you most so Phoenix Newsletters are your go-to monthly resource for the latest and greatest Phoenix insights.