At Certara, we offer a comprehensive suite of services designed to progress your drug or therapy to the next stage of maturity. Our expertise spans across all domains of drug development to give you comprehensive insights, clarity and direction to reach success.
Transformative services for drug development
Transform the pace and quality of drug development using services that build on decades of experience.
Contact us
Level up your drug with our services
Driven by extensive expertise in drug development, regulatory affairs, market access, and commercialization, coupled with deep scientific knowledge on a global scale.
Streamline development and drug development support with tailored MIDD-driven strategies across all phases to optimize execution and decision-making.
Reach the right audience at the right time with expert planning and writing support. Our services meet regulators’ expectations and support your submissions in any phase.
Seamless support across every stage of drug development
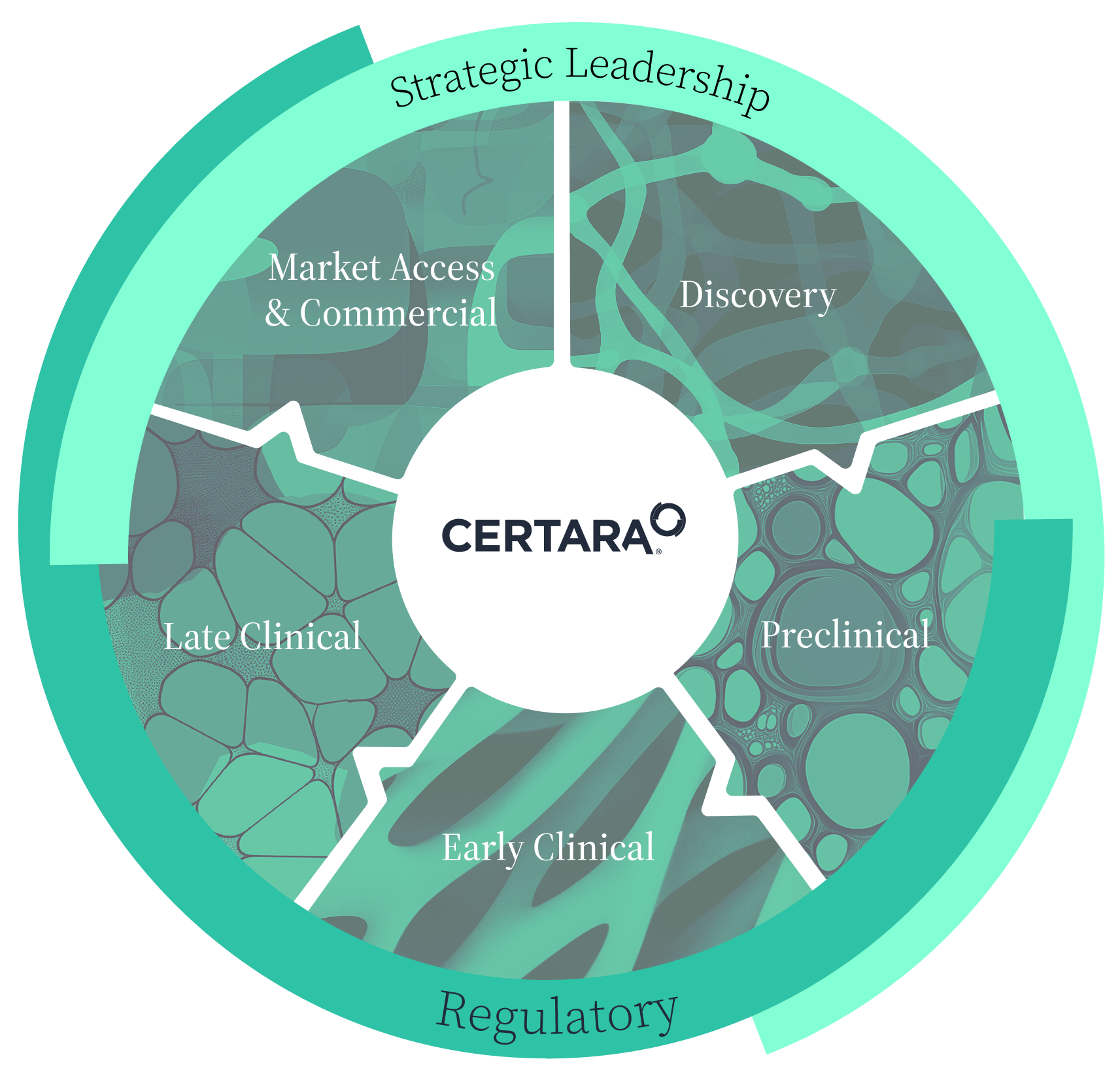
Certara’s integrated services empower you to tackle challenges at every stage of drug development. By uniting expertise across discovery, preclinical, clinical, commercial and market access, and regulatory phases, our vision is that information flows freely, providing you with actionable insights that enhance safety, efficiency, and success.
This holistic approach means that every decision you make is better informed, every development phase supports the next, and your drug achieves new heights. With Certara, you gain predictive, evidence-based strategies, reduced effort, and greater confidence in bringing life changing therapies to patients faster.
Related resources
View allContact us
We know – we have a formidable and comprehensive range of services to help you progress your drug or therapy to the next stage of maturity. Do send an inquiry and we’ll connect you to the expert that can serve you best.
Send an Inquiry
Discover more about Certara
Solutions
Explore how Certara’s integrated services drive success across every stage of drug development. From discovery to regulatory submissions, we deliver evidence-based, faster, and more efficient solutions tailored to your challenges.
Software
Unleash the power of Certara’s industry-leading software. Predict outcomes, optimize processes, and ensure data-driven decisions with tools trusted by global regulators and researchers alike.
About us
Learn more about Certara’s mission to transform drug development for good. With decades of expertise and proven success, we’re your trusted partner in innovation and progress.











