Pinnacle 21 clinical data standardization software empowers clinical teams to standardize, validate, and harmonize data effortlessly. Achieve flawless submissions and accelerate development with confidence.
Pinnacle 21
Achieve submission ready conformance with Pinnacle 21 clinical data standardization software

Learn more about Pinnacle 21 clinical data standardization software
Optimize quality & speed from setup through submission with clinical data standardization
Manage and optimize your clinical trial data from the start.
Why implement clinical data standardization software?
The Pinnacle 21 clinical data management software platform transforms clinical data into submission-ready deliverables – enhancing data quality across studies, and validating datasets against global regulatory standards.
By integrating metadata management and continuous data validation in a collaborative platform, Pinnacle 21 speeds timelines, reduces risk, and ensures data integrity throughout the clinical data flow.
Metadata management for eCRFs and submissions.
Centralize metadata for eCRFs, data transfers and submission datasets, and harmonize in line with clinical data standards, such as CDISC, as well as your own in-house standards.
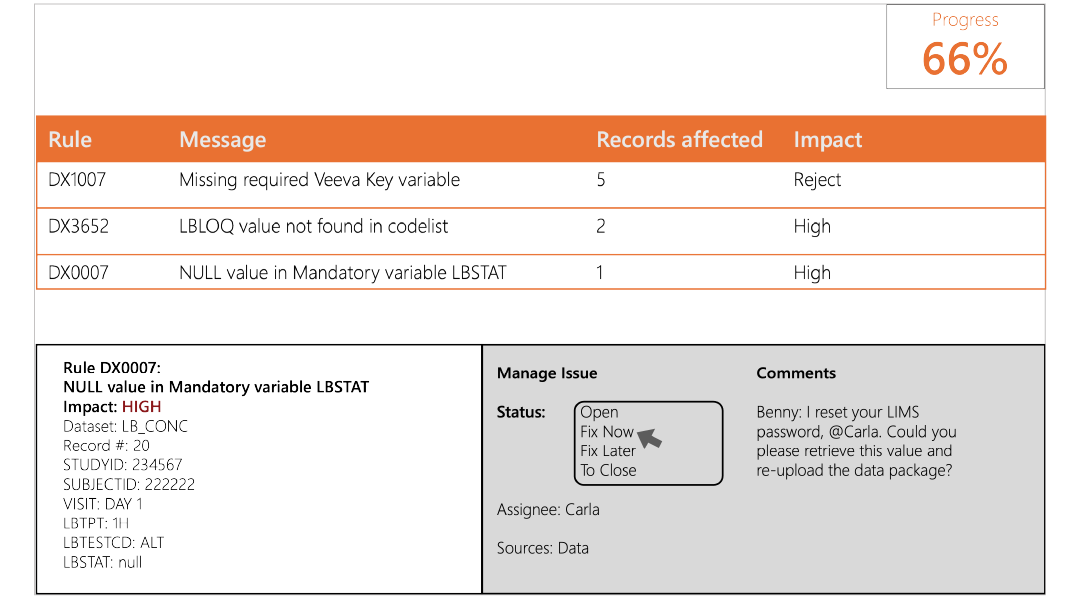
Real-time data validation with issue tracking.
Collaborate with CROs, developers, and others in a central workspace to resolve issues discovered during validation.
Effortless collaboration across both internal and external clinical teams.
Design your transfer specifications with each vendor and accept data packages in the cloud.
Leaders in clinical data management
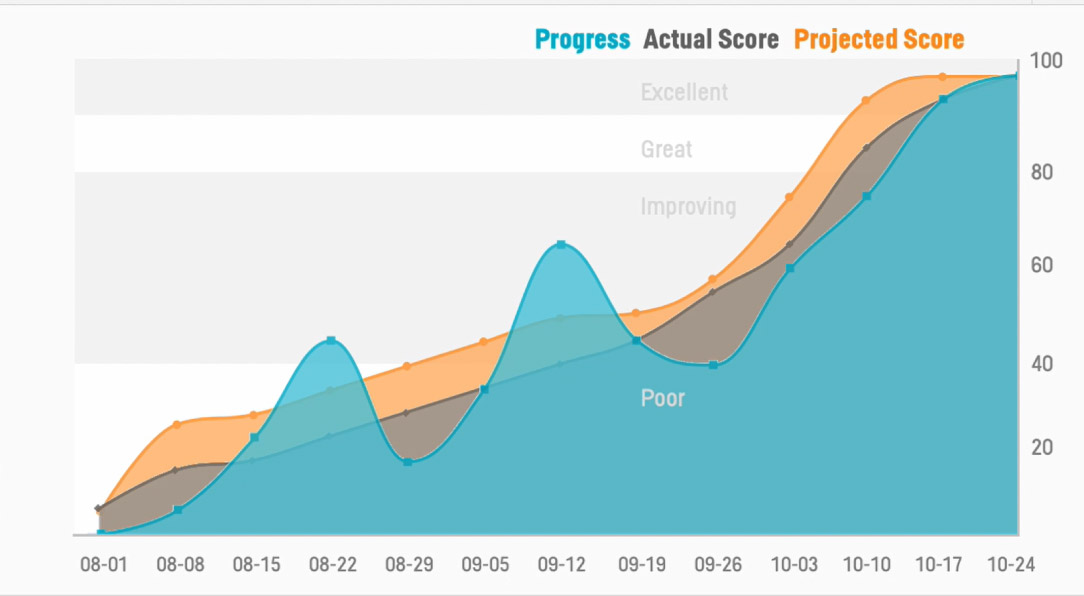
Continuous data validation
Insist on clinical data standardization
Pinnacle 21 for data standardization streamlines metadata management, collection workflows, and external vendor data exchange, helping you manage and optimize your clinical trial data from the start.
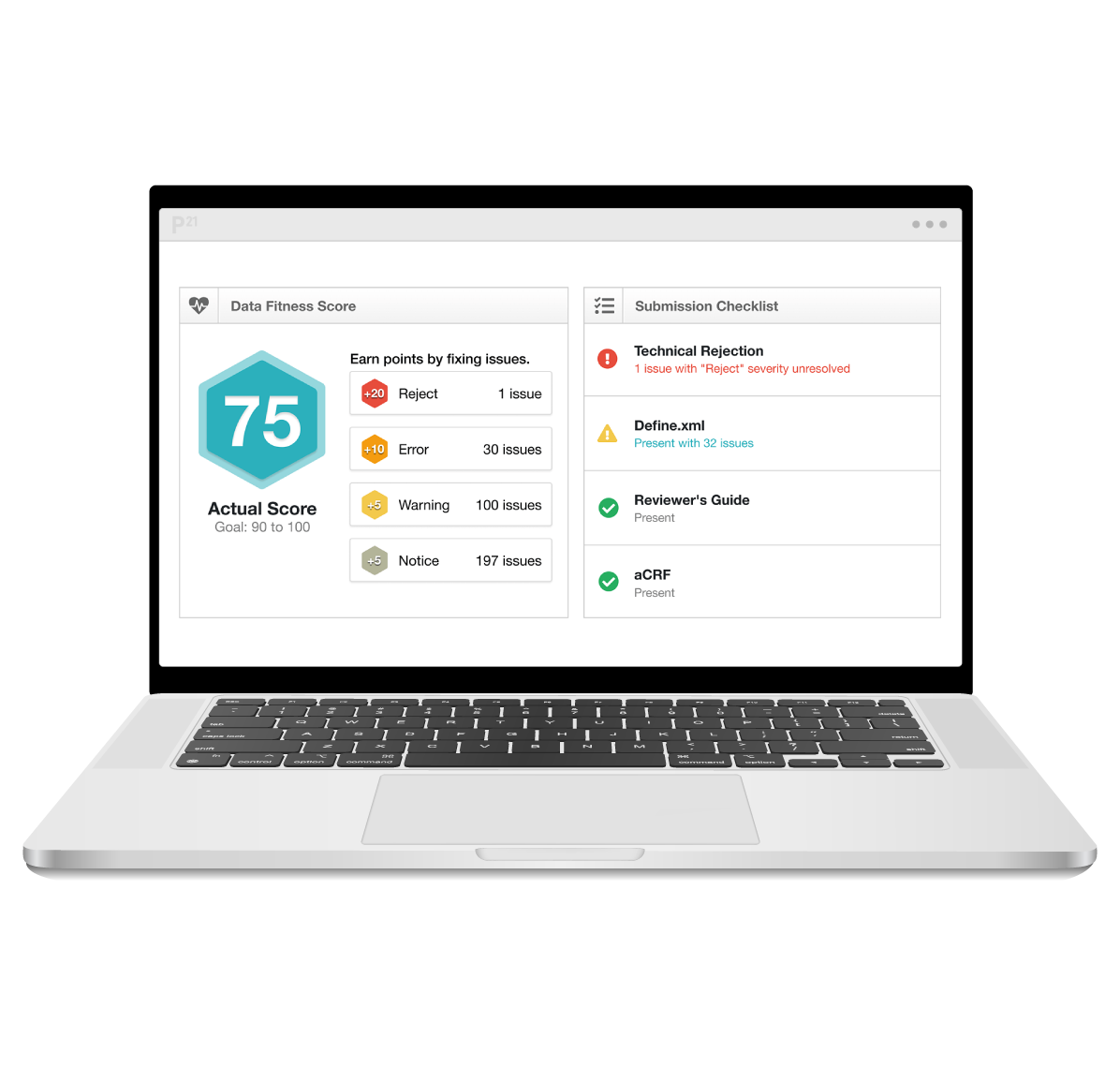
Get fit to submit
Pinnacle 21 for data validation shortens the time to analysis and submission-ready CDISC datasets, define.xml files, and Study Data Reviewer’s Guides.
Pinnacle 21 Community resources
Support from our technical experts
Need a hand preparing your SDTM datasets or submission deliverables? Our technical support team combines expert knowledge of clinical data standards with decades of experience helping thousands of clients achieve regulatory compliance and expedited submissions.
Data standardization and validation resources
View all
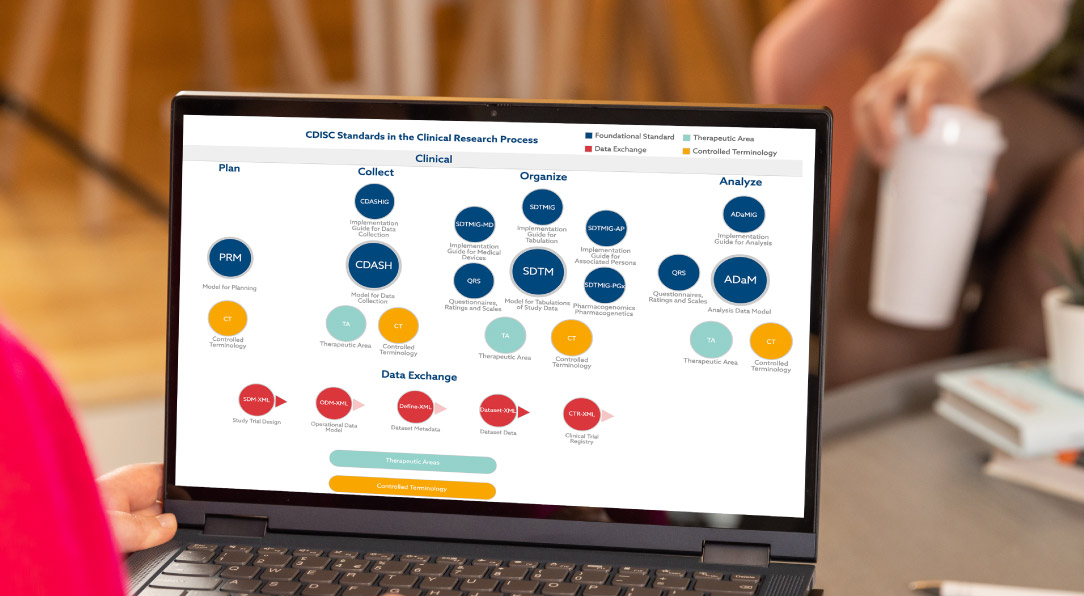
Aligned with CDISC data standards
The Clinical Data Interchange Standards Consortium (CDISC) develops data standards. Many regulatory agencies require drug submissions to conform to CDISC data standards. Pinnacle 21 embraces all CDISC guidance.
Transform clinical data with Pinnacle 21
Schedule a consultation to see how Pinnacle 21 clinical data management software can accelerate your path from study design to submission.