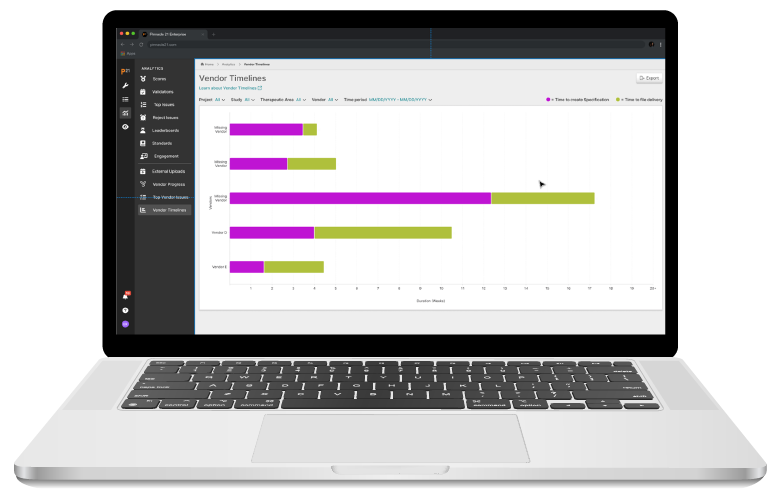
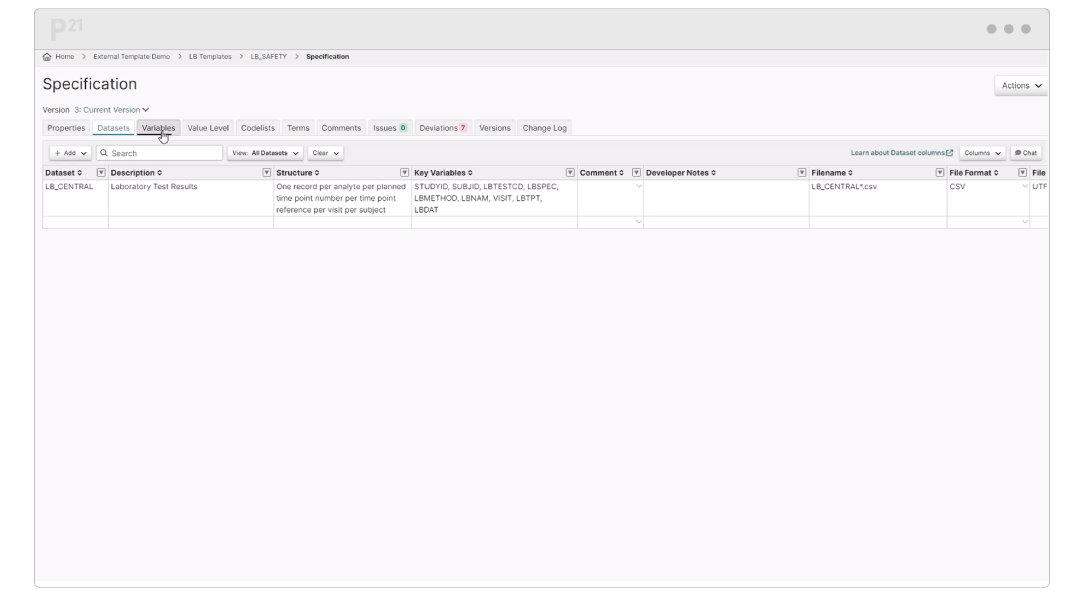
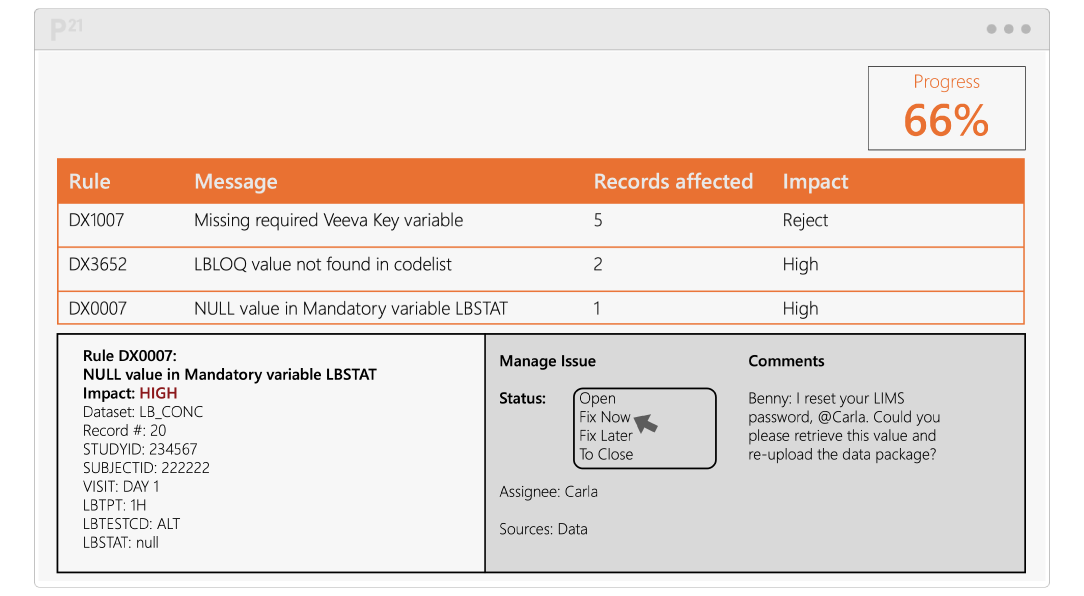
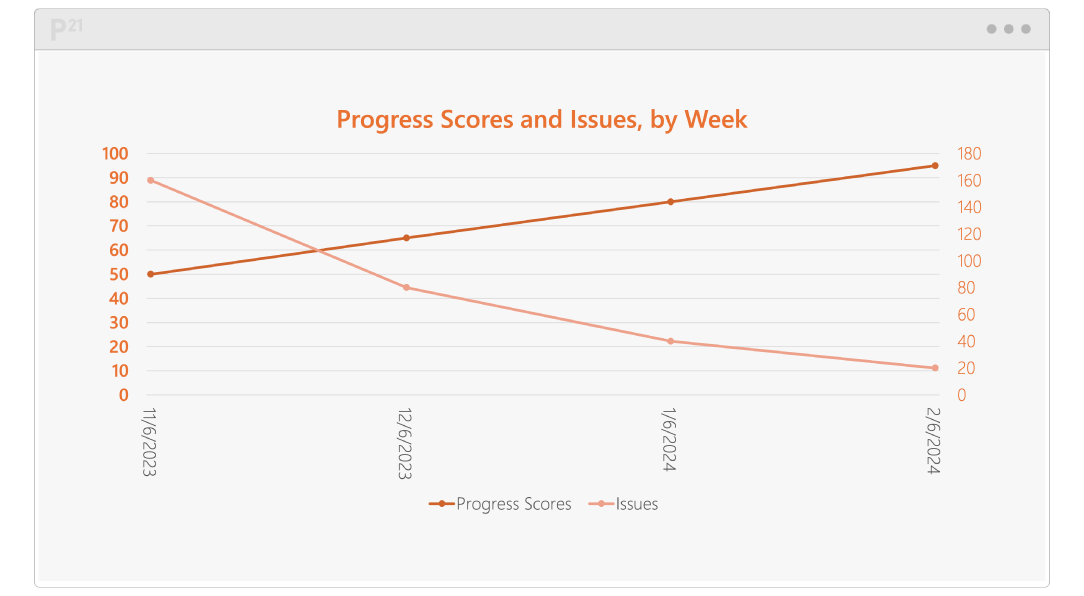
Pinnacle 21 Enterprise (P21E) revolutionizes the way you manage non-CRF data by providing a centralized, collaborative platform for managing external data.
With non-CRF data now accounting for over 70% of clinical trial data, traditional manual methods of relying on spreadsheets and email chains are no longer efficient. P21E ensures high-quality, compliant data from external vendors through real-time validation, collaborative issue resolution, and a transparent audit trail.