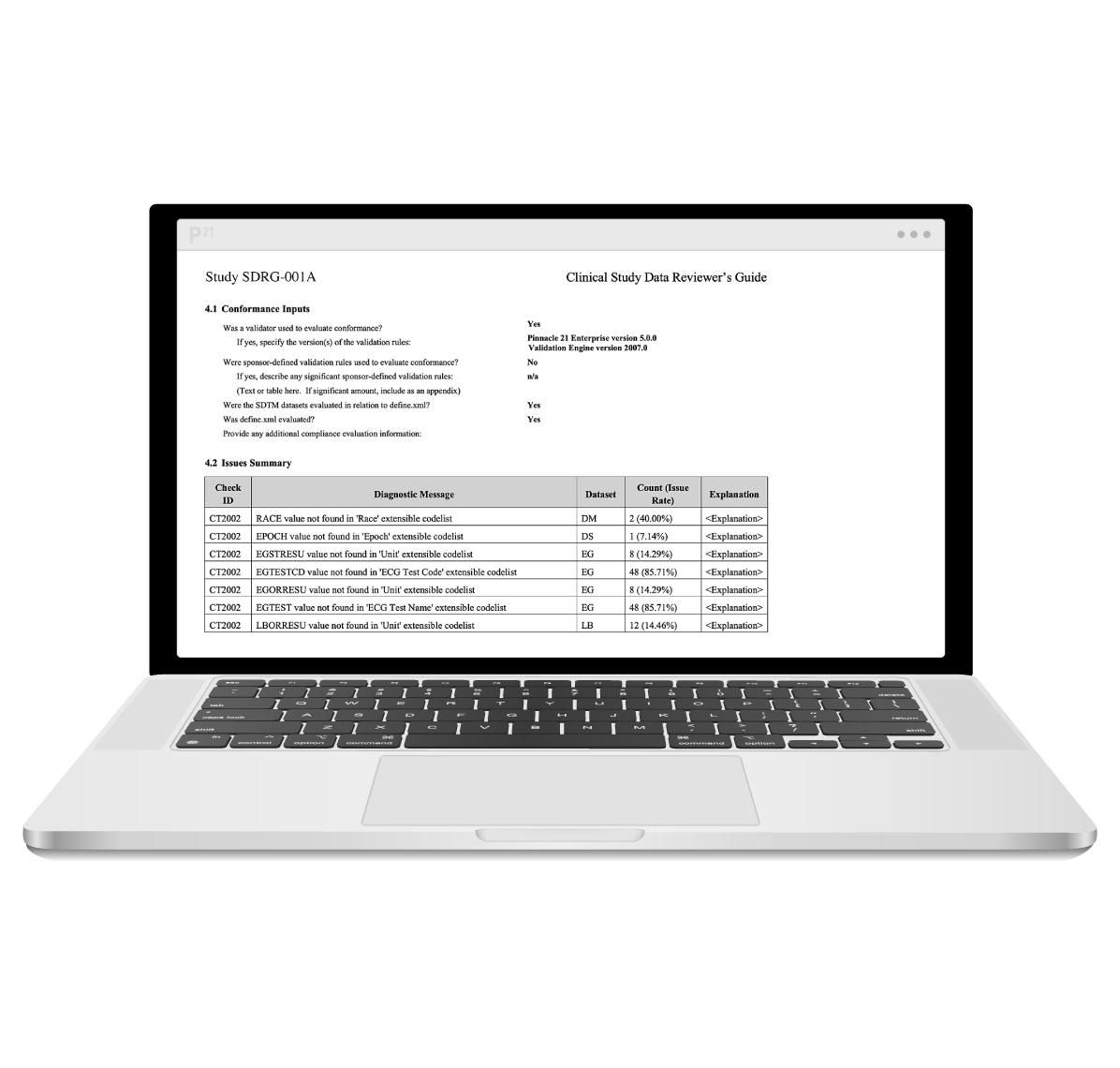
Regulatory agencies rely on clear and detailed documentation to navigate clinical data submissions. The Study Data Reviewer’s Guide (SDRG) provides essential context and clarity, expediting the review process.
However, manually compiling and formatting this guide can be time-consuming and prone to errors. Pinnacle 21 Enterprise revolutionizes this process by automating content aggregation and formatting, allowing you to generate a complete SDRG with just one click.