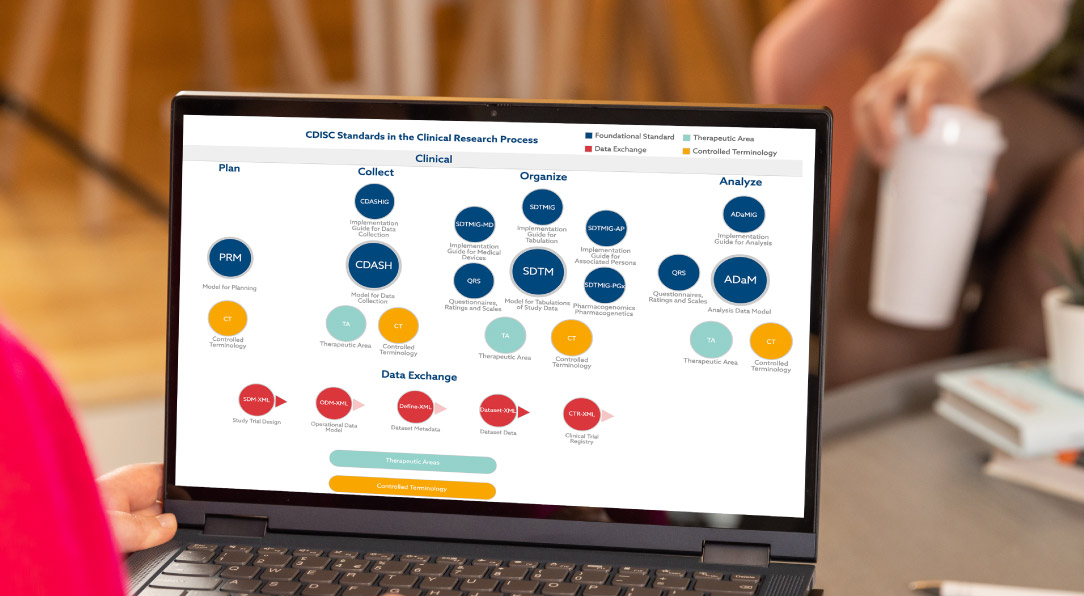
Define.xml is a critical component of regulatory submissions for SDTM, SEND, and ADaM datasets. However, creating these files manually is time-consuming and prone to errors. Pinnacle 21 Enterprise simplifies this process by automating the creation of define.xml files, dataset metadata, annotated CRFs, and Reviewer’s Guides. With built-in validation and intuitive tools, you can ensure your submissions are accurate, compliant, and ready for approval.
Pinnacle 21
Define-XML: Automate your Define.xml files with Pinnacle 21

Learn more about automating Define-XML
Have your define.xml files ready to submit faster
80
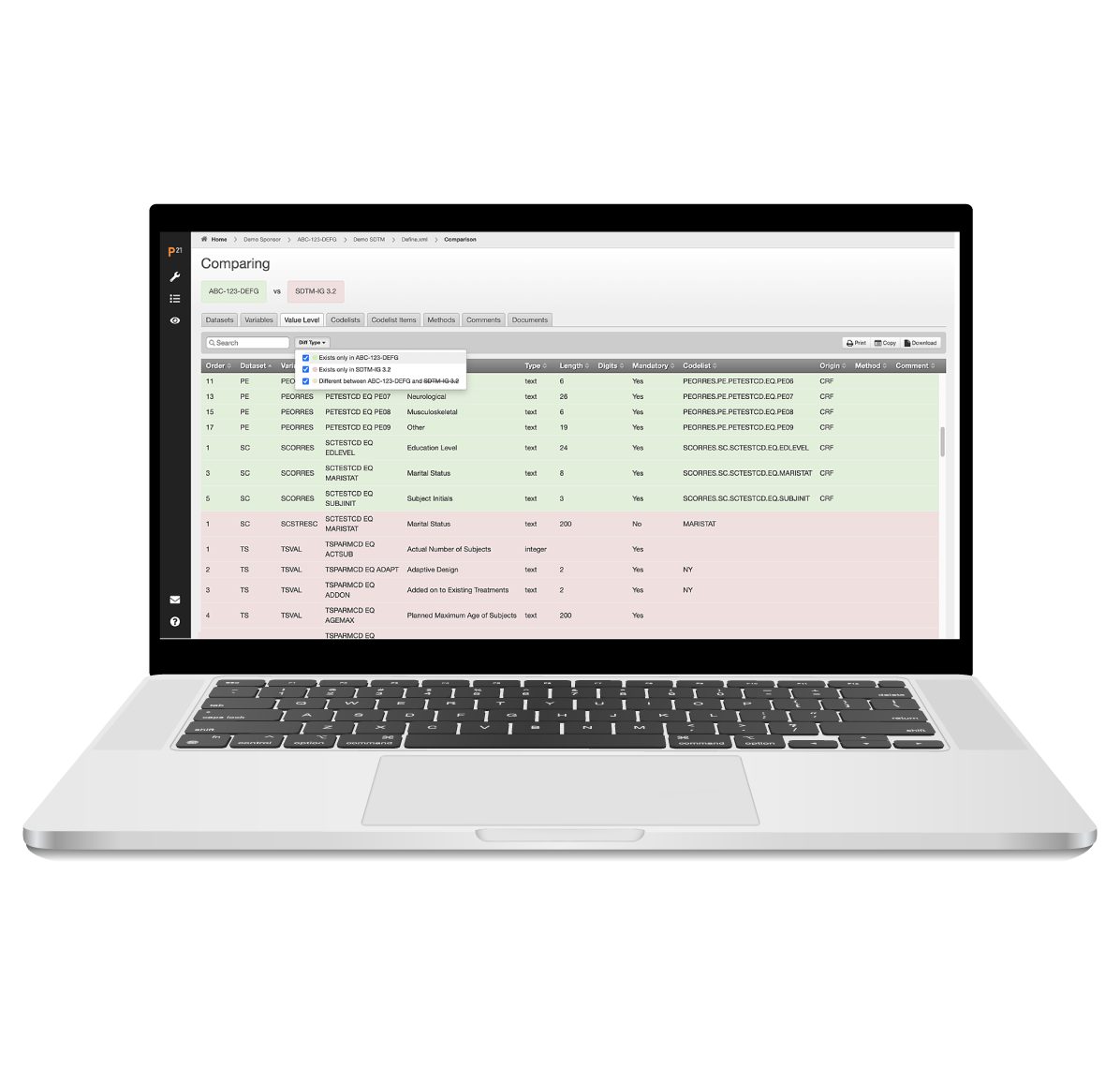
Effortlessly create define.xml files for SDTM, SEND, and ADaM datasets
Pinnacle 21 Enterprise’s define-xml tool is designed to streamline your submission process, ensuring compliance and efficiency. By embedding the Define-XML standard into dataset design, the platform reduces manual effort and eliminates costly errors.
Automate define.xml
Automatically create define.xml files for SDTM, SEND, and ADaM. Simply edit and manage files like a spreadsheet! You can also import spreadsheets to instantly convert to XML format.
Accurately predict define.xml
When you design forms for an EDC in P21E, the platform accurately predicts the define.xml for the datasets that would be created – based on your form design. That way you’ll have submission deliverables from study startup.
Visualize define.xml
Preview your define.xml metadata in PDF, or using the published CDISC stylesheet. This includes full definition of the define, page links to annotated CRFs, and hyperlinks to submission datasets.
Ensure compliance with real-time validation
Any errors or invalid entries are instantly highlighted in real-time. So validation is baked in from the start, and you can be confident your define.xml is flawless! Even import existing spreadsheets, SDTM or ADaM datasets into the platform and validate in real-time. All that’s left is to extract your final define.xml file at the click of a button!
Manage version control
You can update and version your Define.xml files in P21E, allowing complete visibility and change control. Assess changes over time by comparing studies, study versions, or comparing content with standards.
Generate Reviewer’s Guides
Submit clear, complete data and easily share context and pre-empt questions in P21E. Our tool automates content aggregation and formatting, allowing further time savings. Then it’s just one click to generate your submission ready Reviewer’s Guide!
How you’re better off with Pinnacle 21 Enterprise
Related products
Technical support with define.xml
Got an urgent deadline or need a hand producing your define.xml submission package? Our experienced technical experts are here to help prepare your submission ready data, ensuring quality and compliance.
Why Certara
Certara empowers drug development companies and professionals with innovative solutions that help ensure compliance, accelerate timelines, and enhance outcomes.
Trusted by the FDA and PMDA, Pinnacle 21 Enterprise instills confidence that your clinical data is submission-ready. With unmatched CDISC expertise and years of experience in clinical data standards, we deliver industry-leading professional services and support to customers across the globe.

Related resources
View all
Your data is safe
Your data is safe with Define-XML. Pinnacle 21 Enterprise adheres to the highest security standards, ensuring your clinical trial data is protected.
Book a free no-obligation demo
Discover how Pinnacle 21 Enterprise can transform your clinical trial submission process. Book a no-obligation demo today to explore the benefits of automated define.xml creation.
FAQs
What is define.xml?
Define.xml is the metadata file that describes the content and formatting of clinical trial submission datasets. It is a required component for regulatory submissions.
What is the Define-XML standard?
The Define-XML standard is based on the CDISC Operational Data Model (ODM) XML schema. It ensures vendor-neutral and platform-independent data exchange.
Why is define.xml important?
Define.xml ensures that regulatory agencies can understand and review your clinical trial data accurately and efficiently.