November 3, 2025

Ready to streamline your CRF design?
Download our CRF Design Checklist to ensure your forms are clear, compliant and efficient.
✔ Ask the right questions.
✔ Avoid data-capture errors.
✔ Nail the layout that supports high-quality trial data.



Customer Success Manager
Gilbert joined Formedix, now part of Certara, nearly ten years ago as a technical writer. The system knowledge he gained from content development, together with his existing customer service skills, marked him out for transition to the Professional Services (PS) team.
Gilbert has worked with the PS team for over four years, providing both CDISC-based training and software training, as well as support and consultancy services to Pharmaceutical, Biotechnology and Clinical Research Organizations. He helps organizations build studies faster and to a higher quality by making their clinical trial design and regulatory submissions far more efficient.
Today, as Customer Success Manager, Gilbert’s focus is to ensure customers maximize the benefits they can achieve by overcoming their challenges and achieving their goals.
FAQs
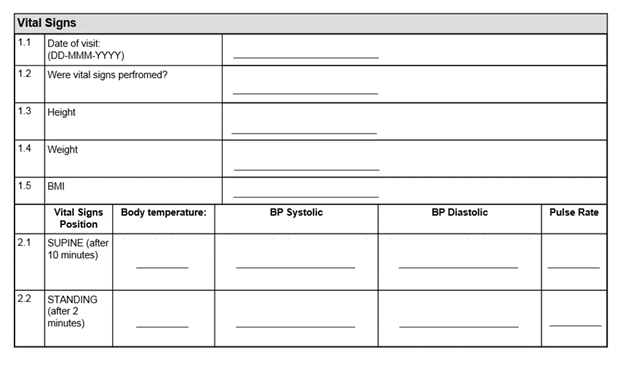
Why is CRF design important?
Good CRF design ensures data accuracy, completeness, and regulatory compliance, reducing errors and delays during trial analysis and submission.
How can CRF design improve data quality?
By using standardized terminology, minimizing free text, and providing clear guidance, CRFs reduce ambiguity and improve the reliability of trial data.
Should CRFs always use SI units?
Yes, using SI units ensures consistency and avoids conversion errors during data analysis and submission.
What are common mistakes in CRF design?
How can you build a case report form and avoid common pitfalls? Over-collecting data, excessive use of free-text fields, duplication of information, and lack of clear guidance are common pitfalls that slow down trial workflows.
Learn more about faster study design & build
Pinnacle 21’s CRF Creator platform gives you the tools to design and build full EDC studies with maximum efficiency and compliance.





