Successful early clinical trials depend on strategic design, informed planning, and a clear clinical development roadmap. Certara helps you optimize First-in-Human (FIH) study design, clinical trial protocols, and development plans to minimize risk and improve decision-making. By leveraging our expertise in profiling drug behavior, anticipating variability, and refining trial design, we empower you to enhance success rates and accelerate your path to approval.
Move your program forward with confidence
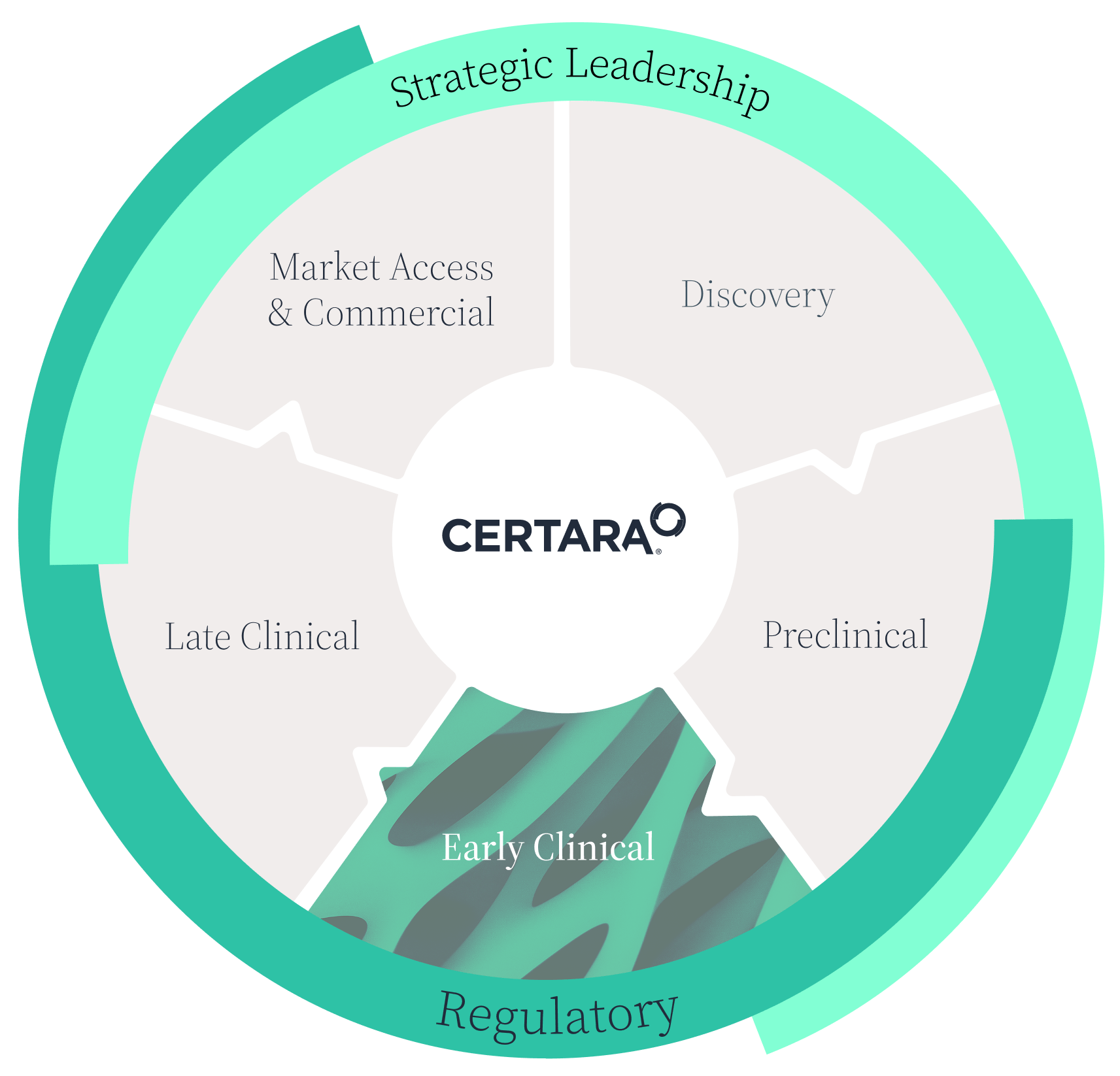
Early clinical

Build your evidence and insights
Explore dosing options
Leverage biosimulation to predict responses, analyze data, and refine strategies to design more efficient early-phase trials.
Robust data insights
Transform complex data into actionable insights through advanced pharmacometric modeling and expert consulting services.
Regulatory readiness
Execute your comprehensive clinical development plan, streamline data validation, and prepare regulatory-ready submissions.

Your trusted guide to early clinical success
Certara’s expertise and world-class biosimulation capabilities optimize First-in-Human (FIH) studies and clinical development plans.
- Refine Dosing & Trial Design with industry-leading platforms like Simcyp Simulator and Phoenix PK/PD to enhance decision-making.
- Strengthen Regulatory Submissions using validated standards for trial design, data analysis, and compliance.
- Gain Expert Insights to confirm dosing strategies, assess drug interactions, and predict outcomes in special populations.
Early clinical resources
View allPartner with Certara for early clinical success
Gain a competitive edge with Certara’s world class biosimulation platforms and expert services tailored to your early clinical challenges.
Send an Inquiry