On October 30, 2024, we hosted our Pinnacle 21 Enterprise (P21E) User Group Meeting (UGM) in Mainz, Germany, as part of the inaugural Certara Certainty Europe customer summit. We welcomed customers from pharmas, biotechs and CROs from across Europe and beyond – including representatives from data standards, statistical programming, and data management, as we aligned on Certara’s mission to:
Accelerate medicines to patients using biosimulation software, technology, and services to transform traditional drug discovery and development
Vision for our clinical study software
We kicked off proceedings by diving into our vision for the P21E clinical study software platform. Attendees were led through the clinical trial data lifecycle – across data management, clinical programming, and biostatistics – and shown how the platform facilitates each stage of the process.
Clinical Data Management
Capture data from forms/EDC and external Vendors
Clinical Programming
Convert data to standard structures, such as SDTM
Biostatistics
Produce stats and figures on safety and efficacy (TLFs)
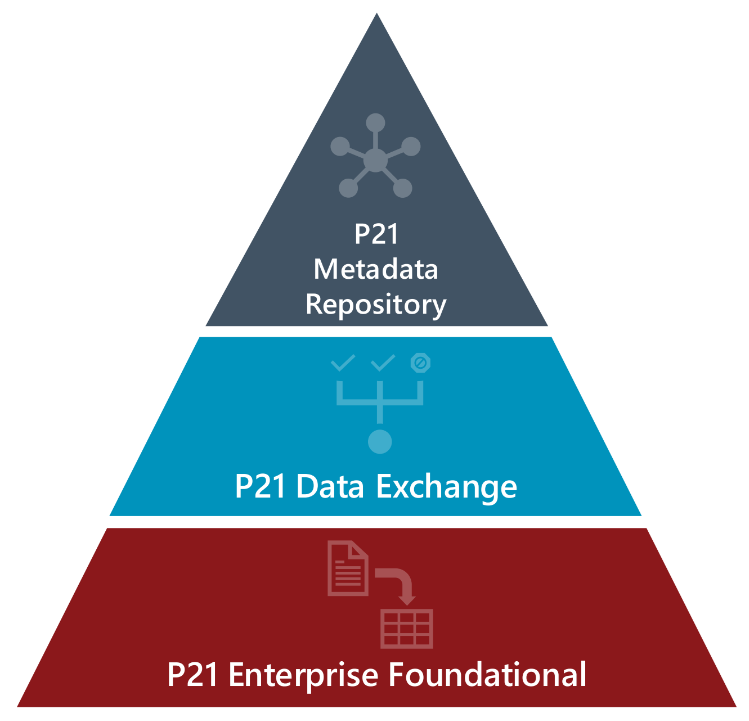
The New Pinnacle 21 clinical study software platform
Since the Formedix ryze Clinical Metadata Repository (CMDR) was acquired by Certara in 2023, the P21 data validation platform has evolved into a data standardization platform, with the goal of achieving:
- End-to-end clinical trial optimization – through standardization and automation from study design to submission
- A clean data pipeline – through governance and reuse of standards for forms, data transfers, and CDISC datasets
Our new clinical study software platform aims to unite and empower data managers, standards managers, and biostatisticians, with a single source of truth with which to make informed decisions. Data managers gain control, with complete visibility. Data managers can now collect data efficiently and confidently. And biostatisticians can convert data with ease.
Customers learnt about new advanced features within P21, including Data Exchange – the new way to collaboratively reconcile external non-CRF data, faster! We also gave a sneak preview of some upcoming features, including the new Rule Designer functionality in P21E, which allows you to create and test your own rules!
UCB’s metadata journey
We later heard from Matthieu Chaton, Head of Data Standards & Programming at multinational biopharma, UCB. Mathieu shared his experience of implementing CDISC SDTM in an end-to-end environment, using our CMDR as the foundation to drive conversions. Their reasons for selecting our clinical study software were driven by many objectives, including the need to:
- Gain control of the entire workflow, from protocol to SDTM
- Achieve consistency across studies
- Connect CRF designs with SDTM mapping (Mappings done upfront)
- Improve quality and reduce errors
- Reduce timelines
We heard how they’re achieving these objectives with the help of our CMDR standards library, which allows them to build forms with CDASH and CDISC terminologies, create SDTM annotations, and easily handle different versions of CDISC standards.
No code SDTM mapping
Mathieu shared how UCB are using the P21 clinical study software platform to draw on their standards library of SDTM mappings & controlled terminologies, and to automatically extract define.xml and specifications. No coding or SAS skills are required. And they finish by validating standards and studies with P21E’s leading validation software.
Demonstration of the CMDR
The meeting concluded with a walk through of the CMDR. We saw how to define and manage a corporate standards library, how to design EDC studies from standards, and how to map and convert data. Our panel of SDTM experts were kept busy with many technical audience questions, and we followed up with 1:1 demos for interested parties.
Click below to find out more about how the evolving Pinnacle 21 Enterprise clinical study software platform can help you run trials more smoothly and efficiently.



