February 10, 2026

Watch Sr Director of QSP, Doug Chung, talk about how mechanistic modeling captures the interplay of inflammatory pathways and therapeutic mechanisms video highlights in Certara’s IBD QSP model.

For a deep dive into Certara’s IBD QSP model, watch this on-demand webinar.
Learn more about Certara IQ
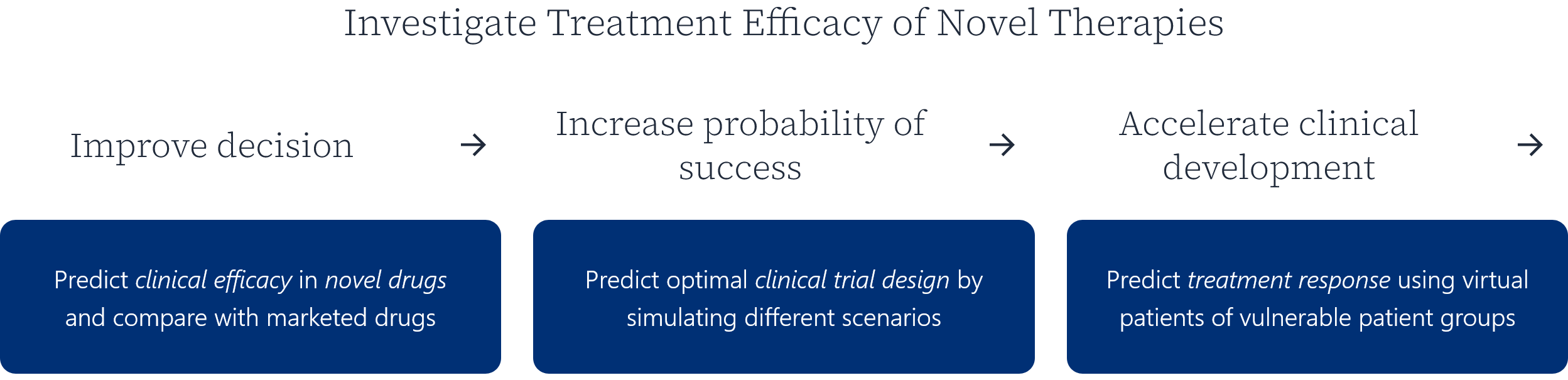
Certara IQ is the AI-enabled QSP modeling tool that will transform your research and scale your molecule’s potential.
Certara IQ offers flexible and scalable licensing options to cater to a variety of users and organization sizes.


Douglas W. Chung, BS, MS
Sr Director, QSPDouglas W. Chung is a highly experienced scientist and consultant specializing in mechanistic modeling to support drug discovery and development. His background is in biomedical engineering and his focus is in quantitative systems pharmacology with over 12 years of experience consulting in biotech and pharmaceuticals. His passion is to grow the field of quantitative pharmacology by expanding diversity in people, fields of expertise, and clinical trial populations.
FAQs
Why is predicting clinical scores important in IBD drug development?
IBD trials are often powered and judged by categorical clinical endpoints, not biomarkers alone. Predicting scores like Mayo and CDAI helps teams:
- assess likely clinical efficacy earlier
- optimize dose and regimen
- prioritize targets and combinations
- reduce late-stage trial risk
How are QSP models validated for clinical decision-making?
QSP models are validated using a learn-and-confirm approach, which typically includes:
- calibration to existing clinical data
- validation against independent datasets
- blind predictions before trial readouts
- sensitivity and uncertainty analyses
This builds confidence that the model can support real development decisions.
How does machine learning complement QSP in IBD drug development?
QSP provides mechanistic explainability, while machine learning enables robust mapping to complex clinical endpoints. Together, they allow teams to translate biological insight into clinically meaningful predictions, without losing interpretability.
See Certara IQ in Action
You May Also Like