October 6, 2025
Why is MIDD so powerful?
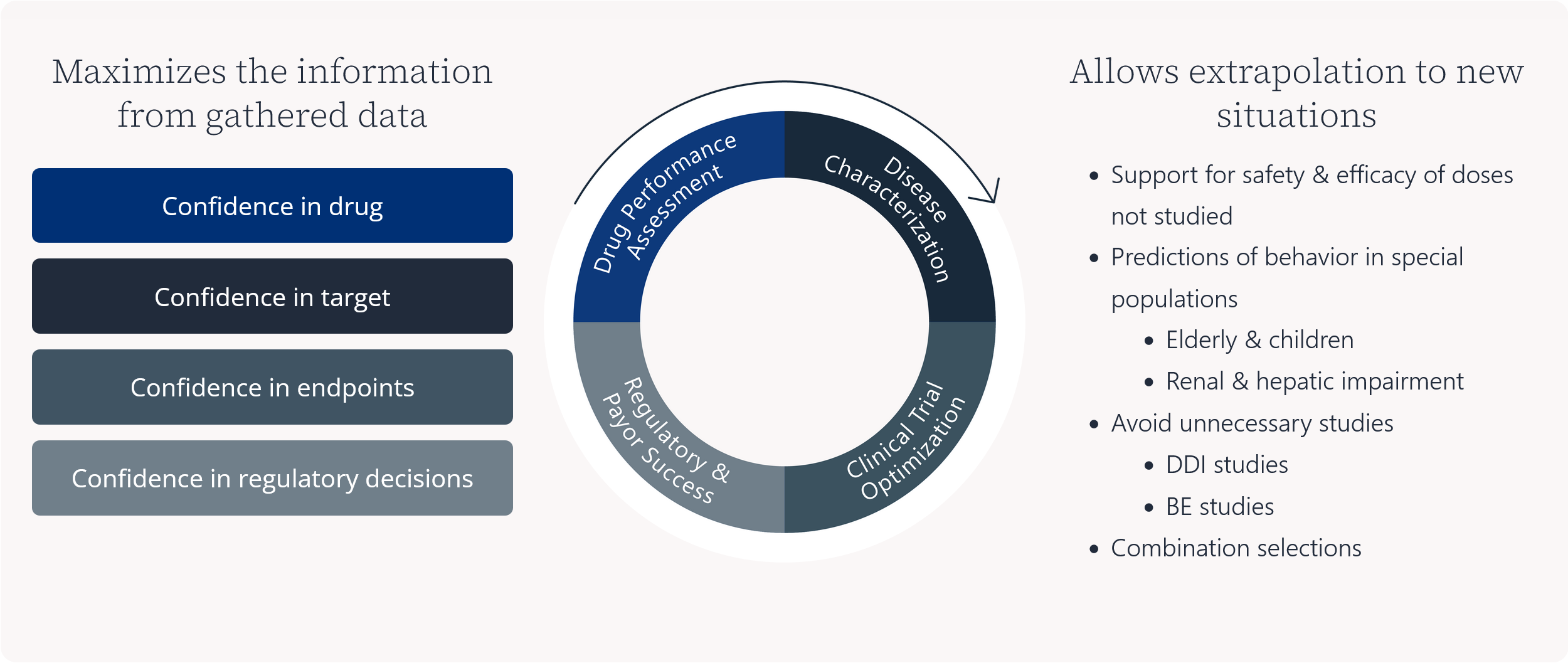
Maximizes the information from gathered data
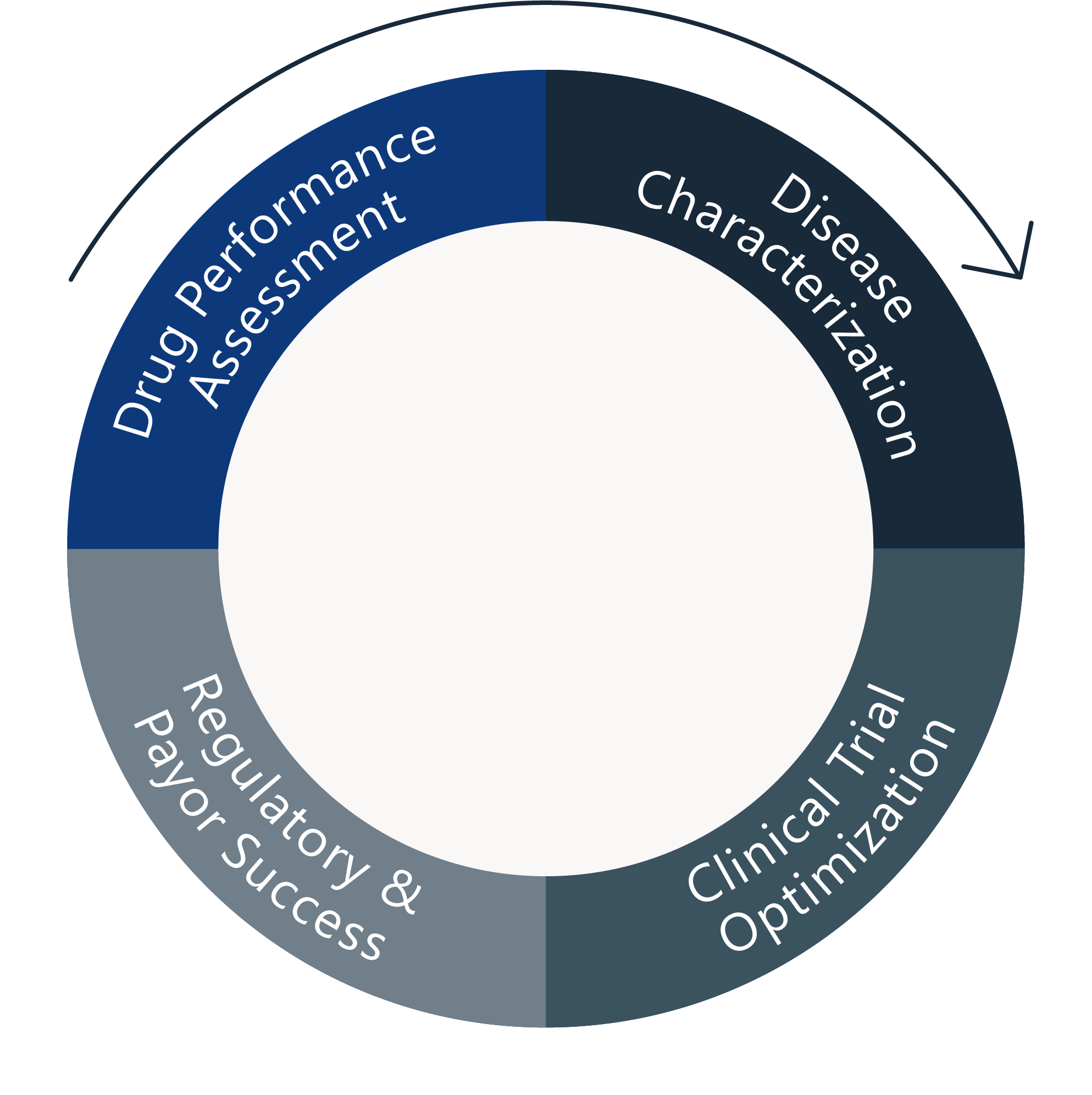
Allows extrapolation to new situations
- Support for safety & efficacy of doses not studied
- Predictions of behavior in special populations
- Elderly & children
- Renal & hepatic impairment
- Avoid unnecessary studies
- DDI studies
- BE studies
- Combination selections
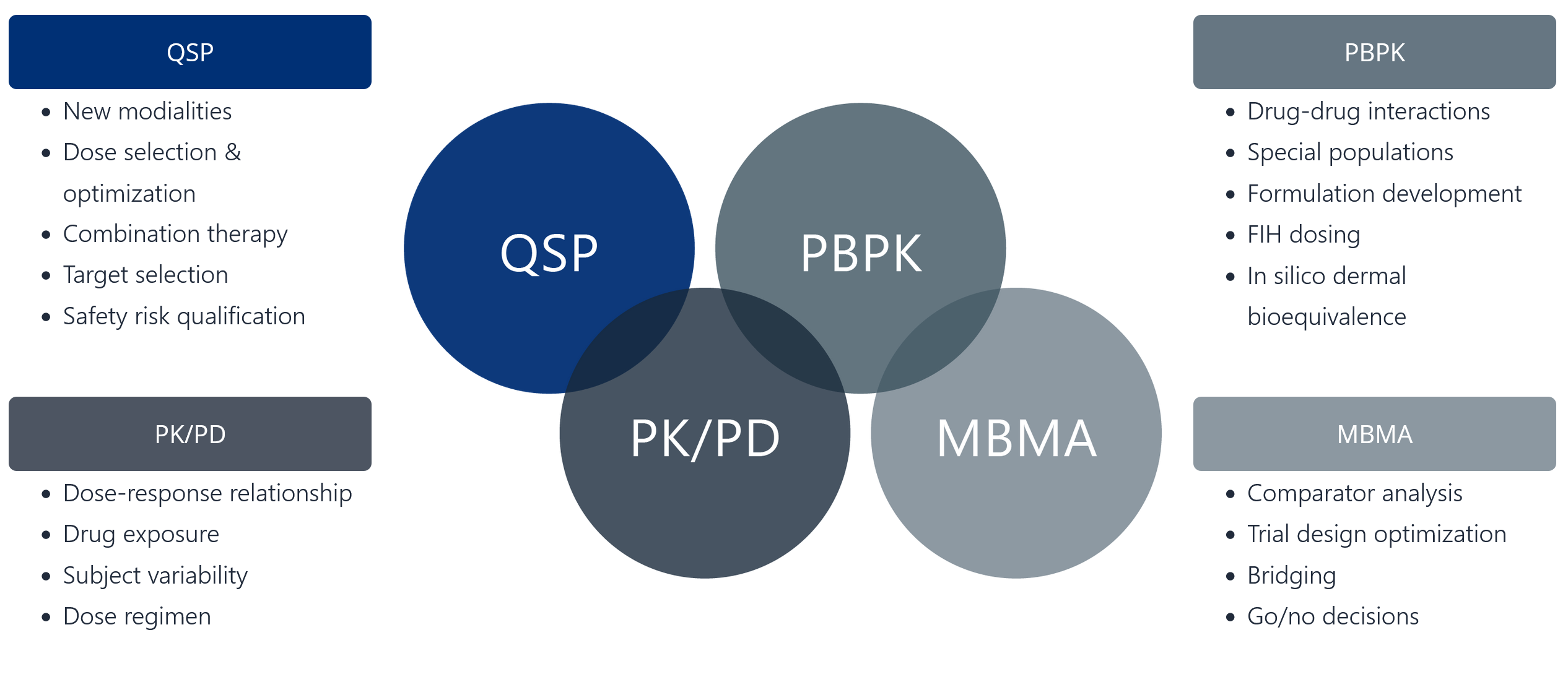
Finding the right tool for the job
- New modalities
- Dose selection & optimization
- Combination therapy
- Target selection
- Safety risk qualification
- Dose-response relationship
- Drug exposure
- Subject variability
- Dose regimen
- Drug-drug interactions
- Special populations
- Formulation development
- FIH dosing
- In silico dermal bioequivalence
- Comparator analysis
- Trial design optimization
- Bridging
- Go/no decisions

To learn how Certara can help you with model-informed drug development services, watch the webinar.
FAQs
What does Pharmacokinetics / Pharmacodynamics (PK/PD) refer to?
PK describes what the body does to a drug (absorption, distribution, metabolism, excretion), whilst PD describes what the drug does to the body (the drug’s effect). PK/PD models link drug concentration to its effect.
What is Population Pharmacokinetics (PopPK) modeling and simulation?
This is a type of modeling that analyzes sources of variability in drug concentrations within and between individuals in a patient population to understand how factors like age, weight, or genetics affect a drug’s pharmacokinetics. The statistical approach uses sparse sampling (i.e. a few blood samples per patient, taken on more than one occasion) which allows the technique to be applied to therapeutic studies.
What is physiologically-based pharmacokinetic (PBPK) modeling?
This is a mechanistic modeling approach that simulates how a drug moves through and is processed by different organs and tissues in the body based on physiological, biochemical, and drug-specific properties. It’s often used to predict drug-drug interactions (DDIs) and outcomes in unstudied populations (e.g., pediatrics, pregnant women, patients with renal/hepatic impairment).
How can MIDD support the FDA's roadmap to eliminate animal testing?
MIDD is key to the shift away from animal testing, offering robust alternatives that align with the FDA’s roadmap and the “3Rs” principle (reduce, replace, refine). For example, techniques like PBPK and QSP modeling use physiological and biochemical data to predict drug behavior, drug-drug interactions, and dosing in humans—without animal testing. By creating virtual models of patients and diseases, MIDD reduces reliance on animal testing while improving the accuracy of preclinical translation. This paves the way for drug development that is faster, cheaper, and more humane.
What is the business impact of MIDD on the pharmaceutical industry?
MIDD has a significant impact on the pharmaceutical industry by streamlining drug development, cutting costs, and speeding up timelines. By combining data from non-clinical and clinical studies, MIDD enables better decision-making and greater certainty across all development phases. For example, a study conducted by Pfizer found that systematic use of MIDD saves an average of 10 months per program. In another study, AstraZeneca found that mechanism-based biosimulation increased the chances of achieving a positive proof of mechanism by 2.5 times, helping to reduce risks and focus resources on the best candidates. Watch this video about how MIDD increases the efficiency of pharma R&D to learn more.

Senior Vice President, Drug Development Science
Dr. Fran Brown is a highly respected professional with proven leadership skills and 28 years of broad experience within pharmaceutical development and due diligence. She has extensive experience with strategic and operational global drug development from early discovery to filing and post-marketing. This experience spans multiple therapeutic areas, small molecules and biologics, global regulatory requirements and registration pathways. She possesses a broad knowledge of product development and portfolio management, with a special focus on development strategy, regulatory interactions and product filings.
Her past appointments include leadership roles within large Pharma as well as in small biotech organizations including head of clinical pharmacology, clinical leader, project development leader, head of clinical operations and due diligence asset assessment. She has over 10 years of experience in providing consulting advice to the pharmaceutical industry and non-profit Global Health Organizations ranging from individual project support, to strategic TA strategy and development planning, portfolio management and corporate transformation. She joined Certara in 2017 and is currently the SVP of Drug Development Science within Integrated Drug Development.

Vice President, Quantitative Science Service
S. Y. Amy Cheung is Vice President, Certara Drug Development Solutions. Dr. Cheung has over a decade of experience working in the pharmaceutical industry at AstraZeneca (AZ), with her role as Senior Pharmacometrician and Project manager of AZ Paediatric working group. She obtained her Ph.D. from the University of Manchester, on the topic of Structural Identifiability Analysis in Pharmacokinetic and Pharmacodynamic Models. After receiving her Ph.D. she worked as a postdoc on mechanistic modeling at the Centre for Applied Pharmacokinetic Research (CAPKR) at the University of Manchester.
She was the co-lead for the cardiac safety training for the IMI DDmoRe project and is also an active member of the EFPIA Model Informed Drug Discovery and Development (MID3) workgroup. She was a chair of IQ Consortium Clinical Pharmacology Leadership Group Pediatric Working Group in 2018 and current co-chair of IQ Consortium TALG, CPLQ PBPK Pediatric group.
This blog was originally published in September 2021 and has been updated for accuracy and comprehensiveness.
Our experts have decades of experience enhancing and optimizing the drug development process
At Certara, we consult with you to address all your drug development challenges. We can streamline every stage of development by providing a tailored solution that combines model informed drug development with expertise across due diligence, CMC, toxicology, DMPK, regulatory strategy, clinical pharmacology, modeling & simulation, biometrics, and more. By uniting data, insights, and expert-driven strategies, we help you deliver new therapies faster to patients.

Contact Certara