Certara’s Secondary Intelligence is a cutting-edge secondary pharmacology software designed to de-risk drug development programs by providing evidence-based insights. Safety issues account for approximately 25% of drug project attrition, often due to secondary pharmacology challenges.
Secondary Intelligence™
Predict and analyze off-target risks in drug development for safer outcomes
Learn more about Secondary Intelligence
Predictive technology for improving safety profiles
Secondary Intelligence revolutionizes secondary pharmacology analysis by assembling, curating, and visualizing secondary pharmacology analyses in one place. It provides up-to-date, literature-based information on expected side effects of compounds engaging with specific off-target receptors. This software enables virtual in vitro to in vivo extrapolation (IVIVE), ensuring more accurate predictions of adverse events and empowering researchers to make confident, data-driven decisions.
- Predict off-target adverse events with minimal input data.
- Prioritize receptor interactions of concern with advanced ranking systems.
- Investigate mechanisms of in vivo and clinical findings with precision.
Benefits of using Secondary Intelligence
Analyze and evaluate likelihood of off-target interaction
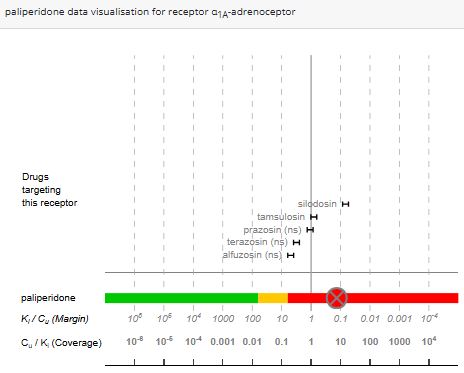
Secondary Intelligence evaluates the probability of off-target interactions during clinical use with precision, presenting results through a clear, color-coded system—red, amber, or green. This ranking is based on a robust quantitative analysis of clinically approved drugs targeting the same receptor, combined with the predicted plasma Cmax of the test compound.
With minimal input requirements, the tool delivers actionable insights. Simply provide the Ki values for each off-target receptor and the predicted plasma Cmax needed to achieve therapeutic efficacy.
Related resources
View allSchedule a demo
Discover how Secondary Intelligence can transform your secondary pharmacology analysis and improve decision-making in drug development.
Contact us
Secondary Intelligence FAQs
What input data is required for Secondary Intelligence?
The software requires minimal input data: the Ki at each off-target receptor and the predicted (or actual) plasma Cmax for therapeutic efficacy. Ki values can also be estimated from % inhibition at a fixed concentration.
How does Secondary Intelligence rank off-target interactions?
It uses quantitative analysis and color-coded rankings (red, amber, green) based on receptor affinity and predicted plasma Cmax.
Can Certara’s team conduct the analysis for us?
Yes, Certara’s experts can perform turnkey secondary pharmacology analysis with a rapid turnaround and provide full support for your program.


