
Xentria is a pioneering biopharmaceutical company focused on developing biologic and innovative drug therapies. Their investigational drug, XTMAB-16, is a novel chimeric anti-tumor necrosis factor alpha (TNFα) antibody that holds promise as a potential breakthrough treatment for pulmonary sarcoidosis.
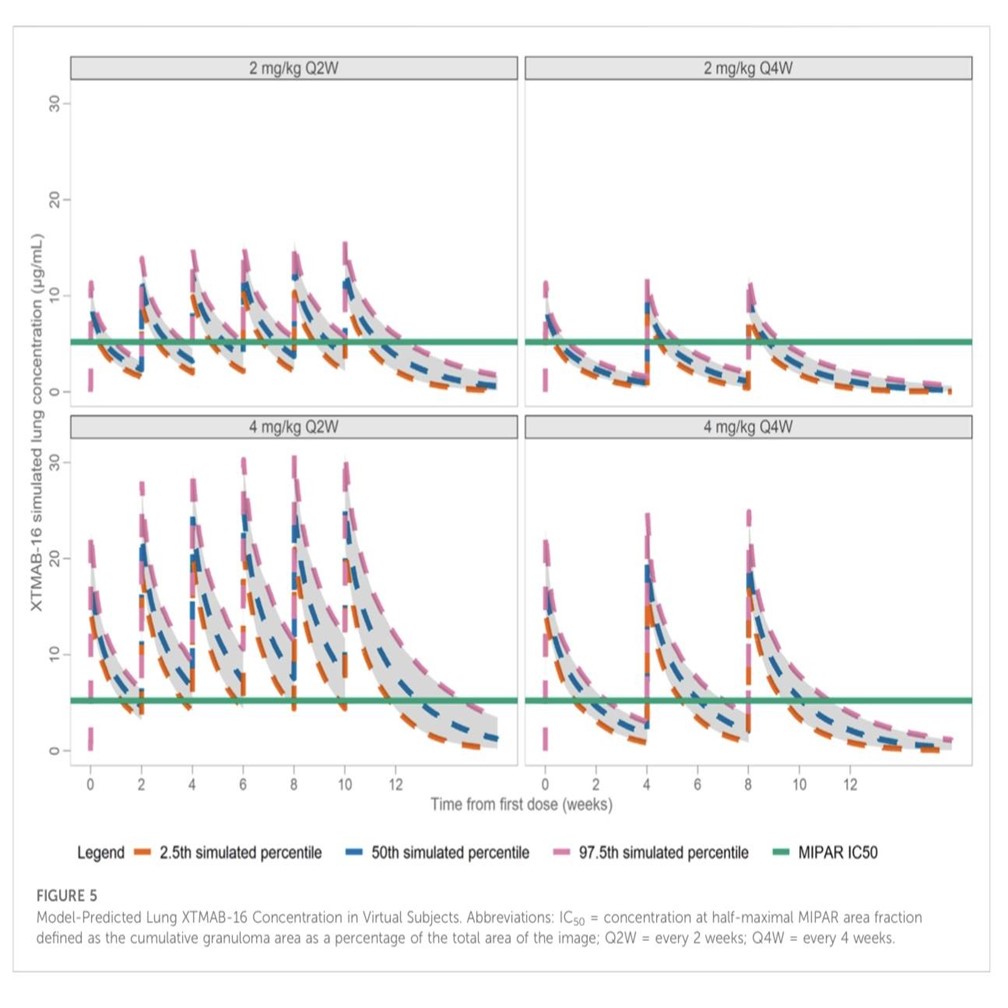
Monte-Carlo simulations were conducted for dosage regimens of 2 and 4 mg/kg over a 16-week period, taking into account factors such as body weight and the presence of ADA. Prediction of pulmonary interstitial fluid XTMAB-16 concentrations were informed by published biodistribution coefficients. Simulations enabled visualization of the XTMAB-16 concentrations at or above the threshold of efficacy estimated from the non-clinical model of sarcoidosis. Optimal dosage levels of 2 and 4 mg/kg administered at specific intervals were determined as a result of the simulations and informed the ongoing clinical development of XTMAB-16 in the treatment of patients with sarcoidosis.
Benefits
Certara played a pivotal role in helping the Xentria team determine optimal dosing strategies for XTMAB-16. These insights provided invaluable guidance for Xentria’s clinical development efforts for this product in a rare indication.
Certara’s comprehensive support, from dose optimization through advanced modeling to expert medical writing assistance, empowers pharmaceutical companies like Xentria to advance their research, make informed drug development decisions, and share their research findings with the scientific community.
- Xentria. Available from: https://www.xentria.com/.
- Certara. Phoenix® NLME. Available from: https://www.certara.com/software/phoenix-nlme/.
- Certara. Phoenix NLME: Population Pharmacokinetic Modeling and Simulation Software. Available from: https://www.certara.com/app/uploads/2020/06/BR_PhoenixNLME.pdf.
- Certara. Top 10 Reasons Why Phoenix NLME Is Your Go-To PK/PD Modeling & Simulation Software. Available from: https://www.certara.com/app/uploads/Resources/Brochures/BR_Top10ReasonsWhyPhoenixNLME.pdf.
- Certara. Scientific and Medical Communications and Publications. Available from: https://www.certara.com/regulatory-science/scientific-and-medical-communications-and-publications/.
- Certara. Scientific and Medical Communications Brochure. Available from: https://www.certara.com/app/uploads/2020/08/Scientific-and-Medical-Communications-brochure.pdf.
- Offman E, Singh N, Julian MW, et al. Leveraging in vitro and pharmacokinetic models to support bench to bedside investigation of XTMAB-16 as a novel pulmonary sarcoidosis treatment. Front Pharmacol. 2023;14:1066454. Published 2023 Mar 20. doi:10.3389/fphar.2023.1066454

To learn more about how Certara experts supported this rare disease drug development program, check out this webinar.
Contact us





