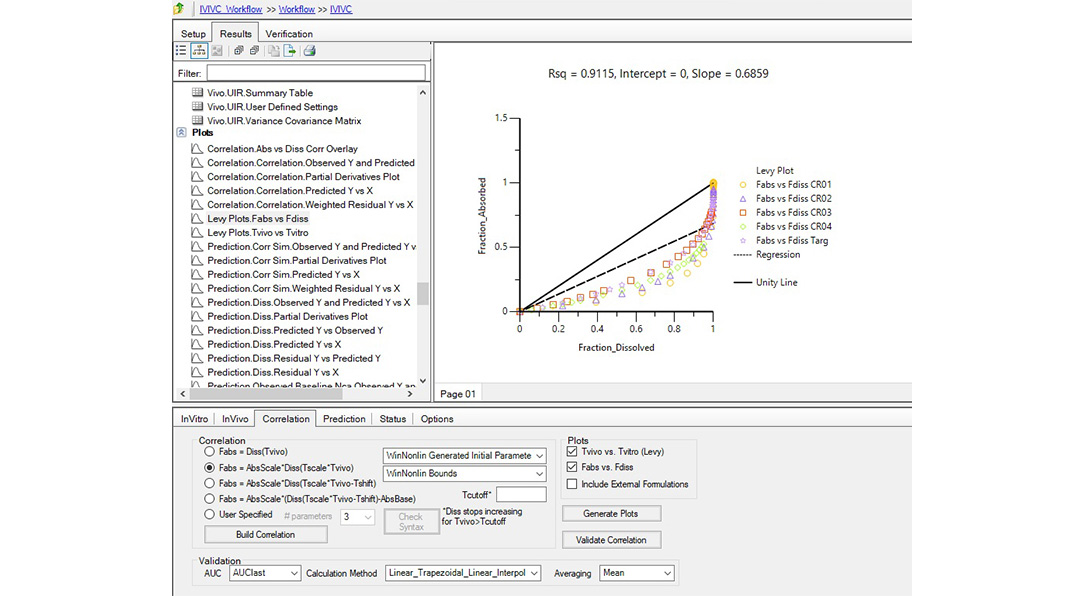
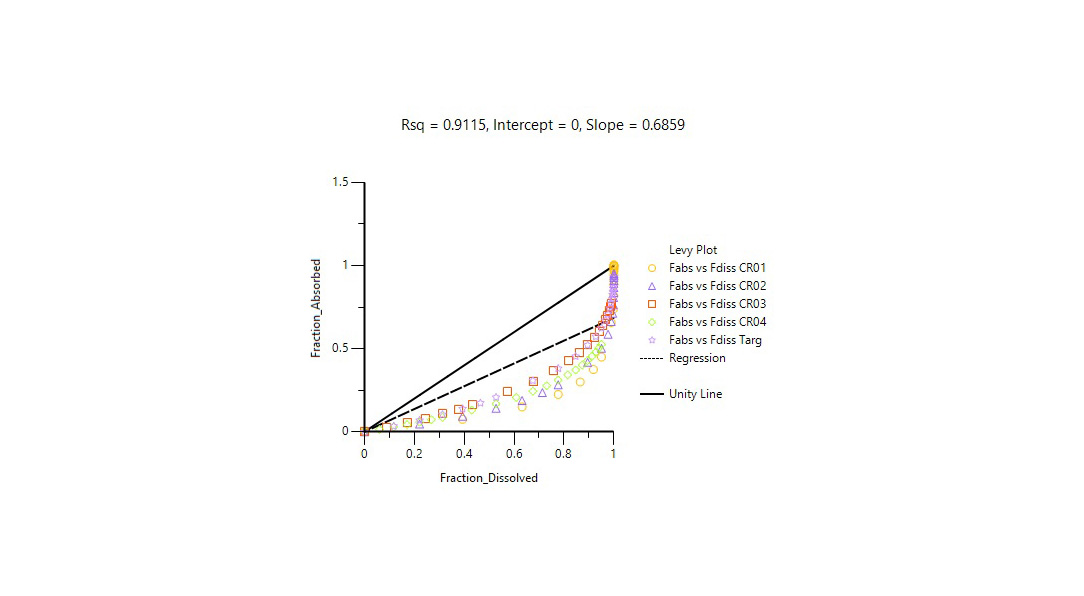
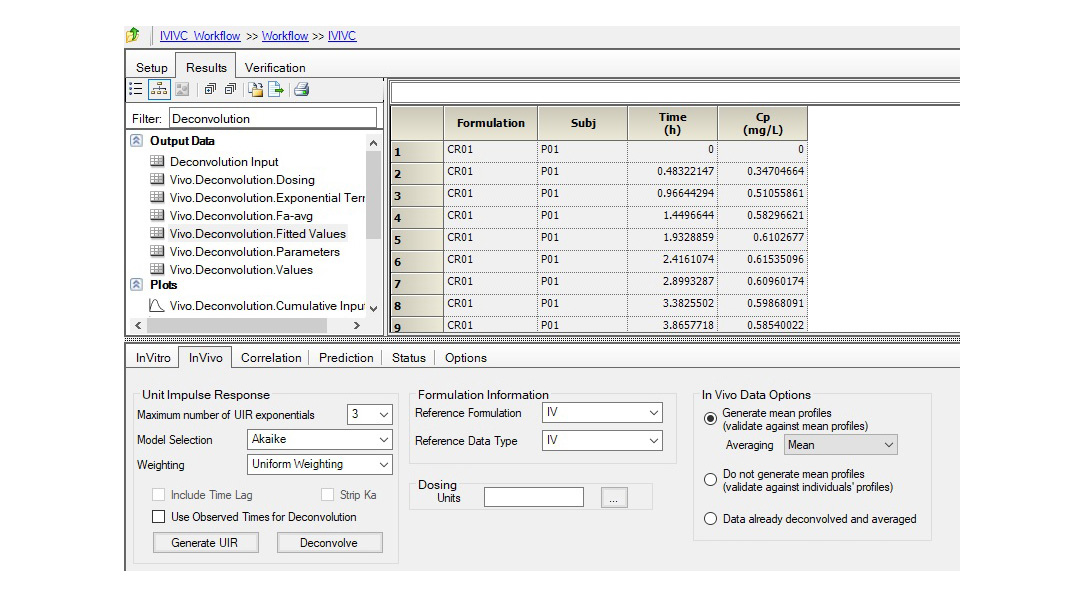
Changes to drug formulations or manufacturing sites can be costly and time-consuming. Regulatory agencies accept in-vitro in-vivo correlation (IVIVC) studies to support biowaivers for SUPAC changes, waive in-vivo bioequivalence for lower strengths, and address other BE requirements.
Certara’s Phoenix IVIVC Toolkit is a leading IVIVC software that provides advanced tools for IVIVC studies, helping scientists improve BE study success with fewer assumptions and better correlation between in vivo and dissolution profiles.