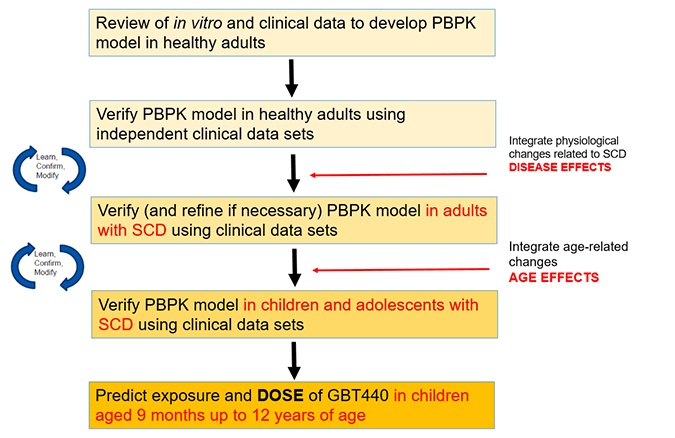
In November 2019, the US FDA granted accelerated approval for Oxbryta™ (voxelotor) tablets for the treatment of sickle cell disease (SCD) in adults and children 12 years of age and older. Voxelotor is an oral therapy taken once daily, it is the first approved treatment that directly inhibits sickle hemoglobin polymerization, the root cause of SCD. Voxelotor was developed under FDA’s accelerated review and orphan designations. As drug types become more complex, we are using physiologically-based pharmacokinetic (PBPK) modeling and simulation to answer difficult development questions, such as in the case of voxelotor. Delivered via multiple pathways, our initial goal was to determine dose projections for children aged 9 months to 12 years which was followed by a request to predict drug-drug interactions (DDI) with CYP3A4 enzymes.
This required us to develop a model using the in vitro and clinical data in healthy volunteers, verify with independent clinical data sets, create a new population file for SCD, and verify with adults and adolescents with the disease in order to predict exposure in children.
For predicting DDI with CYP3A4 enzymes, there were no clinical DDI studies conducted for us to use in building the model. To address this issue, we leveraged the model we built for dose prediction in healthy and SCD patients along with in vitro data to create the DDI predictions. We then performed a sensitivity analyses under multiple scenarios and were able to inform the final drug label without the need for any clinical studies. Further, there was no post-marketing requirement covering DDI.
**Ref: US FDA press release, “FDA approves novel treatment to target abnormality in sickle cell disease,” November 19, 2019
SCD is a group of inherited red blood cell disorders. The most common genetic disease in the world, approximately 250 million people worldwide carry the gene responsible for sickle cell disease and other hemoglobin diseases. Until recently, the only cure for SCD was a bone marrow or stem cell transplant.
Contact us





