
Conference: 2025 ISPE Annual Meeting
Date: August 22 - 26, 2025
Location: Washington, DC
Booth: 303
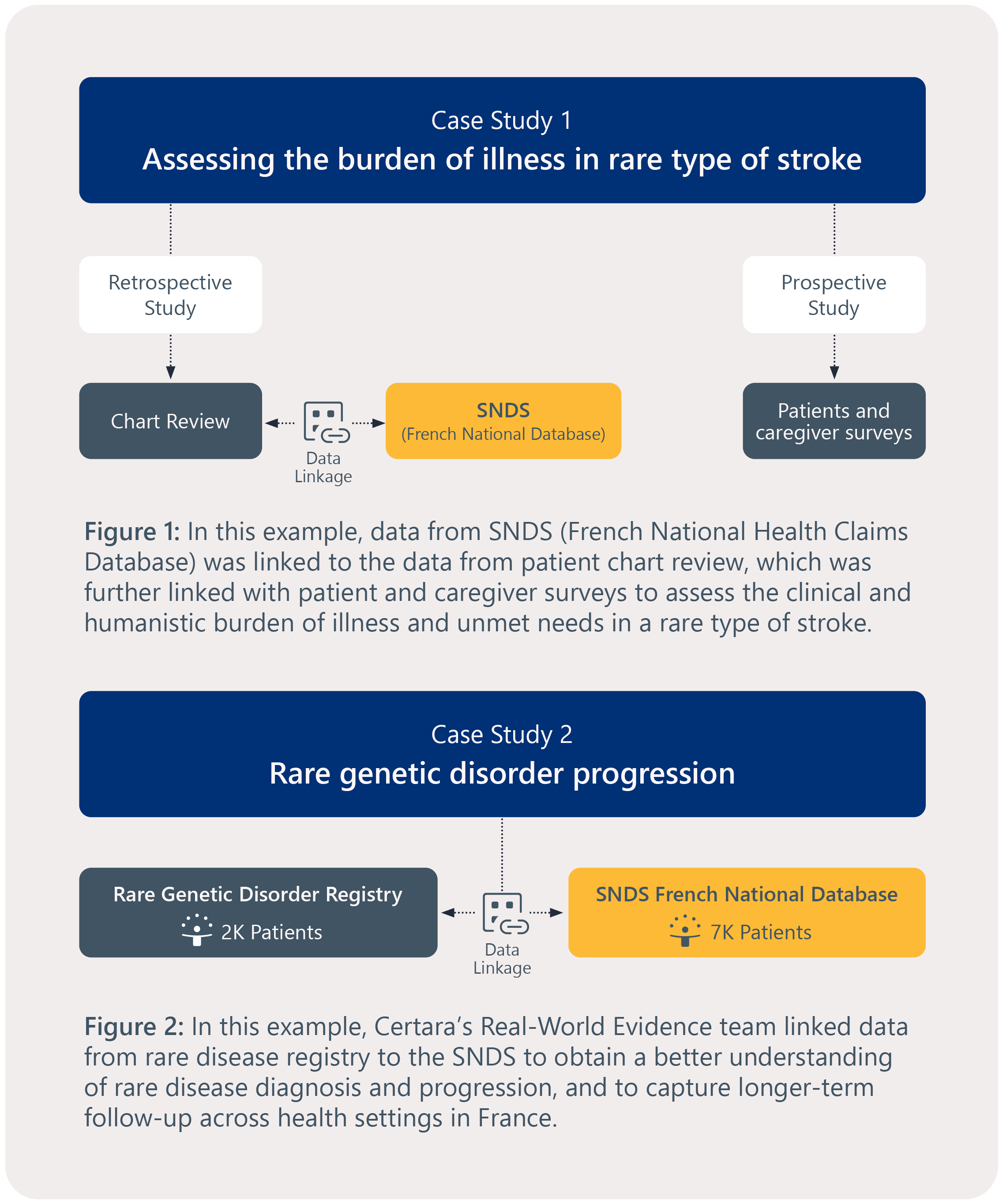
Linking data to solve common yet complex challenges in studying rare disease
Certara’s experts can help you overcome:
- Limited data availability due to rarity of condition
- Locating small and dispersed patient populations
- Incomplete data sources that lack all necessary variables to study, e.g., certain outcomes and resource utilization, but not granular clinical measures or quality of life
Where to hear Certara insights and expertise
Time: 12 – 1:30 p.m. ET
Presenting author: Artak Khachatryan
Co-author(s): Malgorzata Ciepielewska, Aastha Chandak, Alekhya Lavu, Polina DaSilva
Time: 12 – 1:30 p.m. ET
Presenting author: Alekhya Lavu
Co-author(s): Malgorzata Ciepielewska, Aastha Chandak, Polina DaSilva, Gaelle Gusto, Vishnu Sarda, Kalyani Kankanampati, Artak Khachatryan





