July 8, 2025
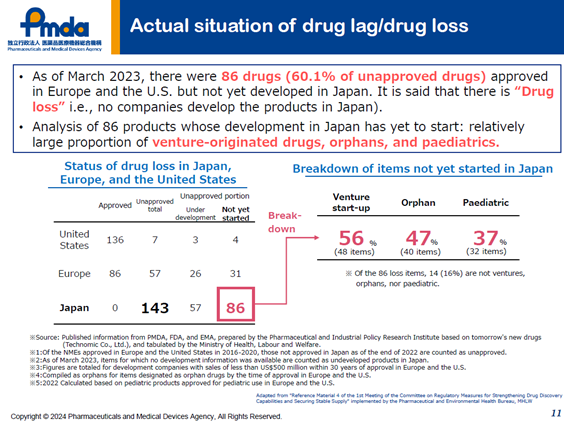
Figure 1: 60% of globally approved new drugs cannot be used in Japan.
(Reference: Daisk Tanaka、Regulatory updates in Japan, 12th Joint Conference of Taiwan and Japan on Medical Products Regulation, 7 October 2024)
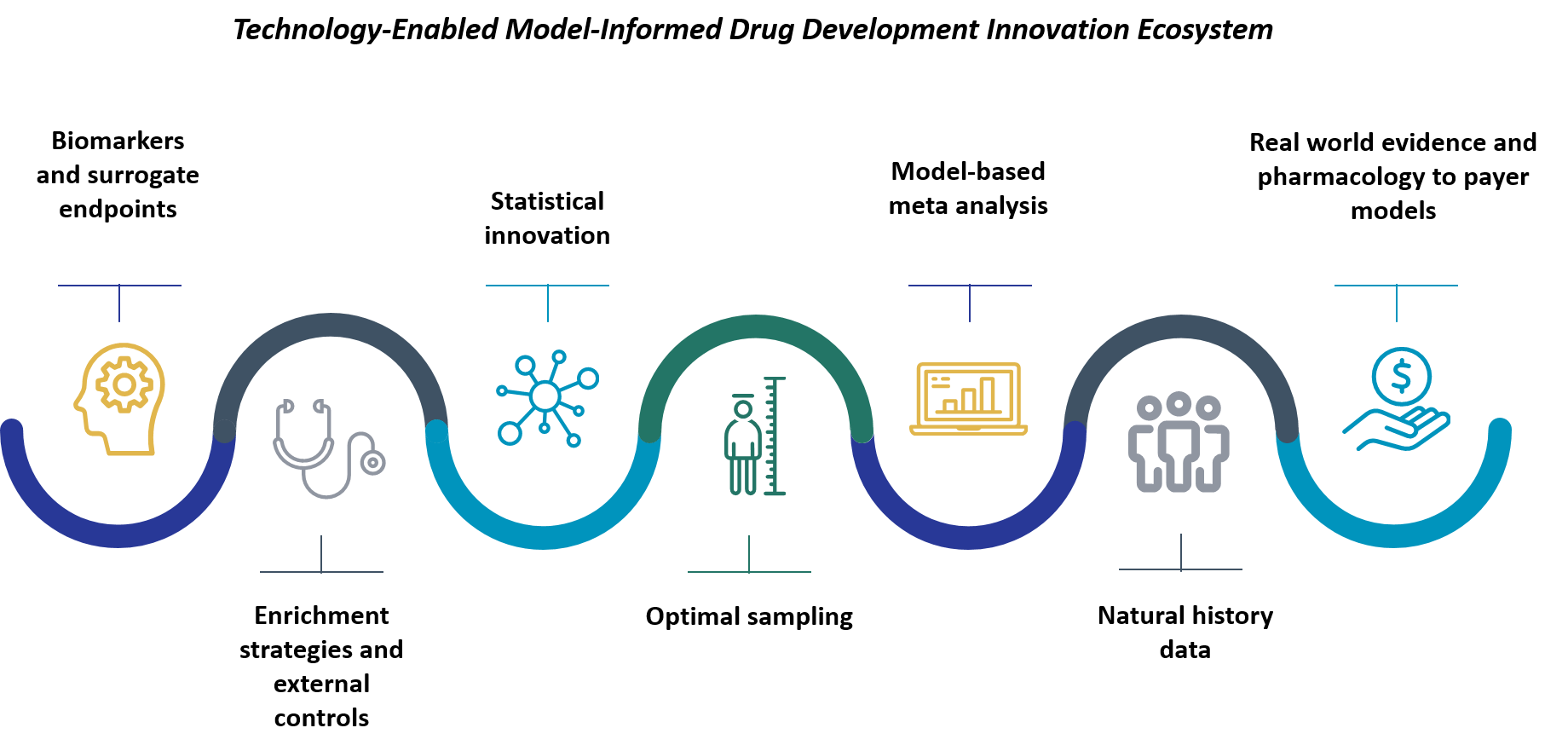
Figure 2: Technology-enabled Model-informed Drug Development Ecosystem
(Reference: Rajesh Krishna, The Utility of Model-Informed Drug Development for Rare Diseases, May 20, 2022 Blog, Certara | Model-Informed Drug Development for Rare Diseases)
Learn more about MIDD and regulatory services for Pediatrics and Rare Diseases for PMDA submissions
Never give up your commercial success in the Japanese market. Our Japanese and global experts will solve your questions and concerns even for the most complex drugs.

References
厚生労働省Press Release 令和6年度厚生労働科学特別研究事業「ドラッグ・ロスの実態調査と解決手段の構築」研究班の整理結果に関する公表、2025/3/31.
医薬品医療機器総合機構(PMDA)理事長 藤原康弘、ドラッグ・ロスに対するPMDAの取り組み、第35回抗悪性腫瘍薬開発フォーラム, 2024/02/17
Rajesh Krishna, The Utility of Model-Informed Drug Development for Rare Diseases, May 20, 2022 Blog, Certara | Model-Informed Drug Development for Rare Diseases
Certara. 4 Ways That Mechanistic Modeling Accelerates Bispecific (and Multispecific) Antibody Drug Development. Blog, Sep 12, 2024.
Certara. Quantitative Systems Pharmacology (QSP) Modeling for Immunotherapy-induced Cytokine Release Syndrome. Blog August 20, 2024 .
Fediuk et al. End‑to‑end application of model‑informed drug development for ertugliflozin… CPT: Pharmacometrics & Systems Pharmacology, 2021.
Hasegawa M, Kijima S. MIDD in Japan- Implementations, challenges and opportunities. Adv Drug Deliv Rev. 2025 May;220:115553. doi: 10.1016/j.addr.2025.115553. Epub 2025 Feb 28. PMID: 40024482.

Senior Consultant, Certara G.K.

Senior Director of Marketing, APAC & EMEA, Certara

Director of Content Strategy, Certara
Contact us





