The acquisitions of targeted radiation therapy companies (TRT) Point Biopharma by Eli Lilly and RayzeBio by Bristol Meyer Squibb have sparked investors’ interest in this therapeutic class. A growing number of companies are now developing TRTs, and GlobalData reports that venture capital deals in the field have grown 550% to $408 million in 2023.1
The TRT regulatory landscape is also evolving with the US FDA’s (Food and Drug Administration) Project Optimus (read more about this oncology drug initiative) and the recently announced EMA (European Medicines Agency) radiopharmaceuticals guidelines revisions.2 Dose optimization, to maximize patient benefits and minimize potential adverse effects, has become critical in the development of TRTs as well as other oncology drugs.
Clinical Pharmacology and Modeling and Simulation (M&S) approaches can support dose optimization and help streamline TRT development.
What’s a TRT?
Radiopharmaceuticals, for diagnostic or therapeutic purposes, may be delivered through an external source (external beam radiotherapy) or by systemic administration of a radioactive compound.
TRTs are designed to deliver cytotoxic radiation selectively to tumors. Radiopharmaceutical therapy involves either:
- direct delivery of the radionuclide to a particular organ without a ligand (unconjugated radionuclide),
- delivery via a targeting moiety (such as small molecules, peptides, or antibodies) conjugated to radionuclides (targeted radioligand therapies) or
- local radiation delivery using either liposomal or nano constructs or glass and resin microspheres (Figure 1).
This blog will focus on therapeutic targeted radiopharmaceuticals (targeted radioligands), defined as the targeted delivery of cytotoxic radiation to tumor cells and/or their microenvironment.
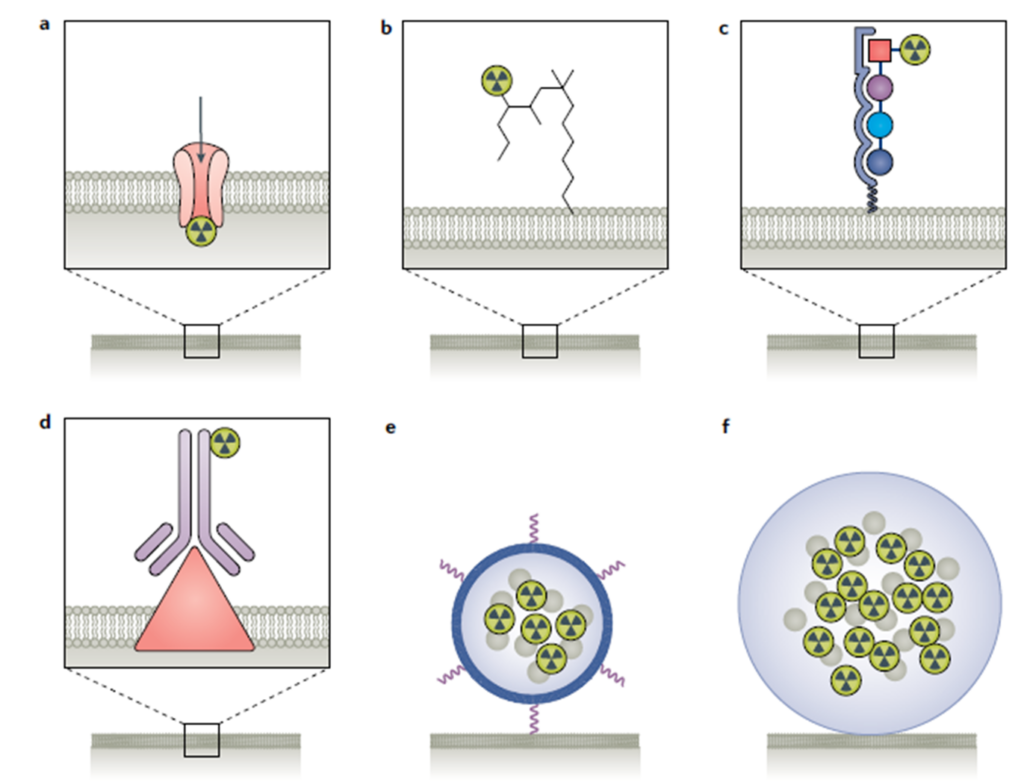
Figure 1 Radiation delivery methods for Therapeutic Radioligands: (a) direct radionuclide delivery to a particular organ (Examples include 223Radium, which is a bone seeker (Xofigo) and Iodine, which is a thyroid seeker); (b) small molecule (examples include 177Lu-PSMA-617 and 131I-mIBG) ; (c) peptide (177Lu-Dotatate); (d) antibody (for example 90Y-CD20, Zevalin); (e) liposomal or nanoconstruct delivery (mainly investigated preclinically); (f) microsphere (examples include TheraSphrere and SIR-Spheres commercially available). Adapted from Sgouros, G., et al. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov 20203
Radionuclides with different emission properties may be used to kill cancer cells. Currently, most therapeutic radionuclides are alpha or beta emitters. Alpha particle emitters (for example: 225Ac, 212Pb) are highly energetic and deposit dense radioactivity over a short range in tissue; while beta particle emitters (for example: 177Lu, 90Y) have a longer range but deposit energy at a lower density than alpha particle emitters.4
Dose optimization of TRTs
Our experience with targeted radioligands, regulatory feedback received by our clients, and relevant FDA/EMA guidances inform these considerations.
Although the January 2023 draft FDA guidance5 on dose optimization for oncology drugs specifically states that it doesn’t address dose optimization for radiopharmaceuticals, recent FDA feedback has shown that the agency still expects dose optimization for targeted radioligands.
In the context of Project Optimus, PK (from both blood radioactivity and the targeted moiety) and dosimetry/biodistribution data are key to supporting dose selection. Appropriate PK assessments help to quantify the TRT’s effective half-life. Likewise, image-based, patient-specific dosimetry allows quantifying the therapy’s biodistribution in tumors and normal organs. Post-therapy imaging for assessing the biodistribution of the TRT is essential to validate that the uptake pattern is as intended and to calculate the absorbed doses in tumors and normal organs at risk, for each radioactive dose level.
Targeting moiety mass dose
TRTs are comprised of a targeting moiety and a radioactive payload, and the dosing needs to be optimized for both. Because the amount of radioactive and nonradioactive materials in the dosing mixture (e.g. specific activity) can affect the TRT biodistribution, the targeting moiety mass dose should also be optimized. The FDA Guidance on Nonclinical Studies for Oncology Therapeutic Radiopharmaceuticals6 emphasizes the need to evaluate multiple dose levels in nonclinical biodistribution studies to optimize organ biodistribution (as shown in Figure 2).
In early clinical trials, as commonly done for imaging agents, assessing the impact of mass dose levels on the tumor-to-background ratio may help to ensure an adequate tumor-absorbed dose while reducing the radioactivity uptake in normal organs, thereby improving the benefit/risk ratio of the TRT and ultimately strengthening the TRT dose justification.7 However, the need for mass dose optimization depends on the biodistribution profile of the drug and the tumour type. For some compounds, the assessment of mass dose effect on tumour absorbed doses may be confounded by inter-patient tumour variability and target expression heterogeneity.
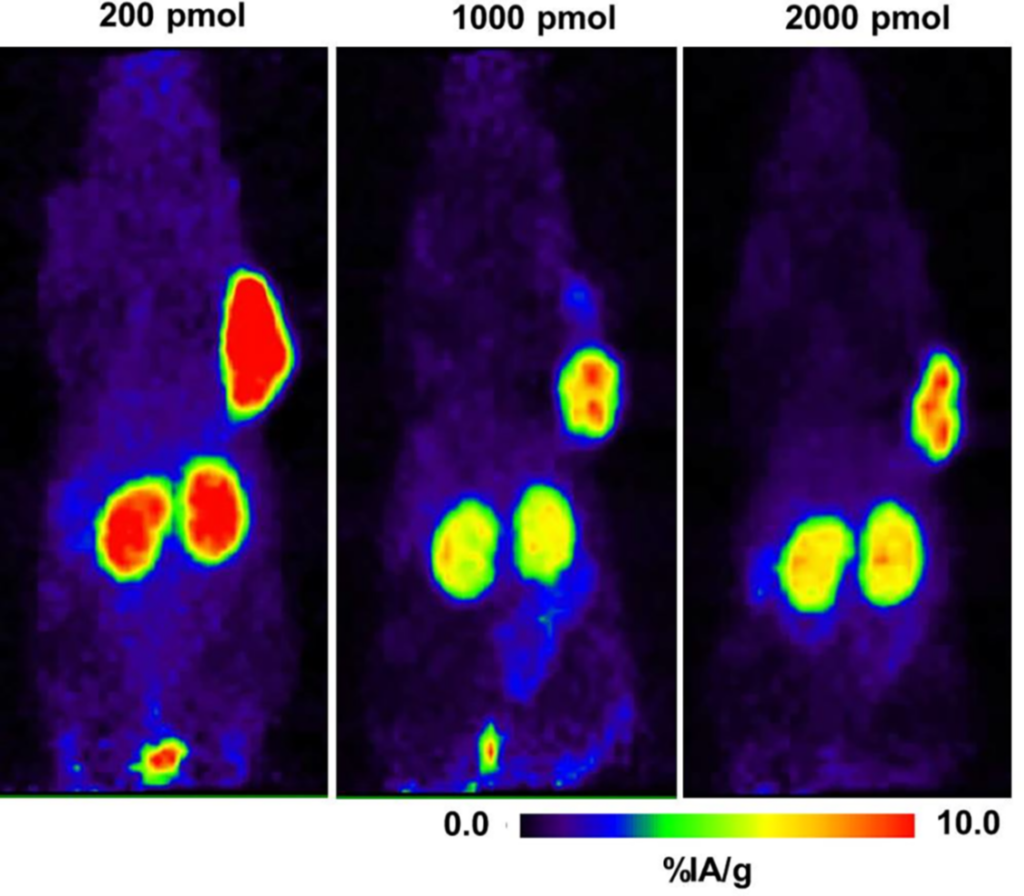
Figure 2. Mass dependence (200, 1000, and 2000 pmol) of the 64Cu-NODAGA-JR11 biodistribution in mice bearing tumor xenografts (%IA/g = percentage of injected activity per gram). Adapted from Rylova SN, et al. (2018) 9
Radioactivity levels
For TRT, both the cumulative administered radiation dose and the radiation dose fractionation should be assessed and optimized. Generating data with different fractionated schema in animal models may help to guide radiation dose fractionation in early-phase trials. The FDA requires optimizing radioactivity levels for therapeutic targeted radioligands based on the totality of evidence:
- Cumulative absorbed doses below recommended thresholds derived from external beam radiotherapy10,11,
- PK, pharmacodynamic (PD), and exposure-response relationships for safety and efficacy,
- Safety, tolerability, and efficacy by radioactivity levels.
Data from dose-escalation cohorts is often insufficient to select a proper radioactivity level for later clinical trials. Thus, further dose exploration should be conducted in patients with adequate follow-up before starting registrational trials. This may include evaluating additional dose cohorts, investigating multiple radioactive levels in an expansion phase, and conducting a randomized, parallel dose-response trial.
Dose optimization may also help support future drug combinations by, for example, reducing the red marrow absorbed dose and thus sparing red marrow reserves for combination with potentially hematotoxic drugs, with other targeted radioligands, or with external beam radiotherapy.12
How can M&S support TRT development? 13
PK and biodistribution data can be integrated to create a pharmacokinetic (PK)-radiokinetic (RK) model to describe the targeted radioligand’s disposition and subsequent uptake in selected tissues. The PK-RK model may accommodate contributions of Anti-Drug Antibody-mediated drug disposition (for monoclonal antibody or peptide targeting moieties), bystander effects, and the effects of patient-specific covariates (body weight; BW, body surface area; BSA, gender, age). Such a semi-mechanistic PK-RK model can support safety and efficacy exposure-response analyses which, in combination with using the model in prospective clinical simulations, ultimately supports an optimized dose selection. Read this blog to learn more about the FDA’s recommendations on dose optimization for oncology drug combinations.
Conclusion
The principles of Project Optimus should be applied to both TRTs and other oncology drugs for dose optimization. Appropriate PK, dosimetry/biodistribution data are key to optimizing the dose and dosing interval for targeted radioligands. Since PK and dosimetry data are generally collected early in clinical phases of TRT projects, clinical pharmacologists and pharmacometricians should collaborate, as early as possible, with dosimetry experts, nuclear medicine physicians, and medical oncology experts or supply managers to ensure proper planning and data utilization throughout development phases. If your TRT clinical development plan needs support, our clinical pharmacologists can help. Check out this webinar to learn more.
References
- Intrigued by Novartis and Lilly deals, investors pour money into radiopharmaceuticals. Fierce Biotech. Published online on 08 Dec 2023. Novartis, Lilly drive investor interest in radiopharmaceuticals (fiercebiotech.com)
- Concept paper on the revision of the Guideline on Radiopharmaceuticals; EMA/CHMP/QWP/298182/2023
- Sgouros, G., Bodei, L., McDevitt, M.R. et al. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov 19, 589–608 (2020). https://doi.org/10.1038/s41573-020-0073-9
- Salerno KE, Roy S, Ribaudo C, Fisher T, Patel RB, Mena E, Escorcia FE. A Primer on Radiopharmaceutical Therapy. Int J Radiat Oncol Biol Phys. 2023 Jan 1;115(1):48-59. doi: 10.1016/j.ijrobp.2022.08.010. Epub 2022 Aug 13. PMID: 35970373; PMCID: PMC9772089.
- FDA Guidance ‘Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases Guidance for Industry’, January 2023
- FDA Guidance Oncology Therapeutic Radiopharmaceuticals: Nonclinical Studies and Labeling Recommendations August 2019
- Kletting P, Schuchardt C, Kulkarni HR, Shahinfar M, Singh A, Glatting G, et al. (2016) Investigating the Effect of Ligand Amount and Injected Therapeutic Activity: A Simulation Study for 177Lu-Labeled PSMA-Targeting Peptides. PLoS ONE 11(9): e0162303. Doi:10.1371/journal.pone.0162303
- Rylova SN, et al. (2018); PLoS ONE 13(4): e0195802. The somatostatin receptor 2 antagonist 64Cu-NODAGA-JR11 outperforms 64Cu-DOTA-TATE in a mouse xenograft model. https://doi.org/10.1371/journal.pone.0195802.g007.
- ICRP, 1984. Nonstochastic Effects of Ionizing Radiation. ICRP Publication 41. Ann. ICRP 14 (3).
- Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys. 2010 Mar 1;76(3 Suppl):S108-15. DOI: 10.1016/j.ijrobp.2009.02.089. PMID: 20171504.
- Gill MR, Falzone N, Du Y, Vallis KA. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017 Jul;18(7):e414-e423. doi: 10.1016/S1470-2045(17)30379-0. PMID: 28677577.
- Kletting P, Thieme A, Eberhardt N, Rinscheid A, D’Alessandria C, Allmann J, Wester HJ, Tauber R, Beer AJ, Glatting G, Eiber M. Modeling and Predicting Tumor Response in Radioligand Therapy. J Nucl Med. 2019 Jan;60(1):65-70. doi: 10.2967/jnumed.118.210377. Epub 2018 May 10. PMID: 29748236.





