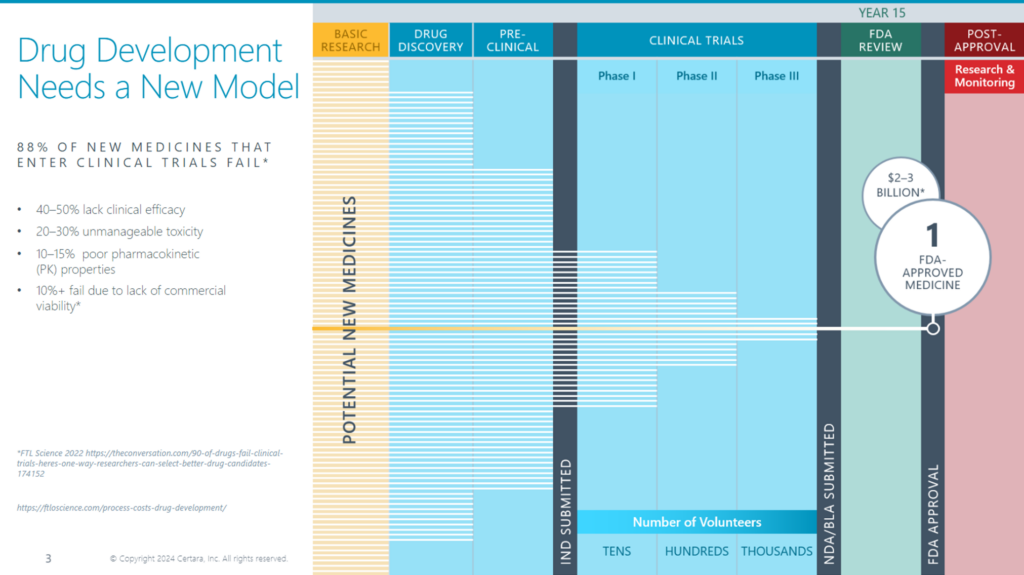
Drug development is a complex, costly, and lengthy process that involves multiple stakeholders, disciplines, and data sources. Even with progress in science and technology, many new drugs don’t make it to market or help patients as hoped. A big problem is that important groups like discovery, preclinical, clinical, regulatory, and commercial don’t work together. These siloes lead to inefficiencies, delays, and uncertainties in the drug development lifecycle.
To address this challenge, Certara, the global leader in model-informed drug development, has launched Certainty, our first annual client summit to bring our community together to share best practices in drug development. Certainty will be held in Philadelphia on May 14-16, 2024.
Whether you use our Phoenix™ PK/PD platform, Pinnacle 21 / Formedix clinical data management suite, Simcyp™ mechanistic modeling software, or our regulatory and scientific services, you’ll benefit from attending this conference. Here are some of the reasons why you should register today:
- Hear from industry experts and thought leaders. Dr. Scott Gottlieb, former commissioner of the U.S. Food and Drug Administration will share his insights on drug development, regulatory policy, and public health in a fireside chat with Certara’s CEO William Feehery. You can ask questions and talk with Dr. Gottlieb and other speakers during the Q&A sessions and panel discussions.
- Hear the Certara vision. Certara scientific and technology leaders will present their vision for a comprehensive solution that combines our software and services to create additional drug development synergies and efficiencies. And see how our other clients have added certainty to their drug programs with “new to you” Certara solutions!
- Learn what’s new for Certara’s products and services. For example, the new Certara.AI platform leverages generative AI to assist with regulatory writing and data analysis. You will learn how Certara.AI can help you automate and streamline tasks such as generating clinical study reports, summarizing data, and creating graphs and tables. You will also see how Certara.AI can enhance the quality and consistency of your regulatory submissions and communications.
- Network with your peers. At Certainty, you’ll have the chance to meet and connect with other pharmaceutical professionals who share your passion and interest in biosimulation. You will also enjoy a fun and relaxing evening social event with your new friends and colleagues.
- Stay updated on the latest pharma trends. You will hear from Certara’s senior management and product leaders, who will share their vision and strategy for the future of biosimulation technology and how it can transform drug development. You will also get a sneak peek at the upcoming software releases and innovations that Certara is working on.
- Get hands-on training on Certara software. Learn how to integrate the latest software features and enhancements into your workflows and processes. You can access live demos, tutorials, workshops, and one-on-one sessions with Certara trainers and support staff. You can also provide feedback and suggestions on improving Certara’s products and services.

Attend Certainty to gain valuable knowledge, skills, and connections to help you achieve better drug development outcomes and advance your career. Don’t miss this chance to be part of the biosimulation community and learn how to increase the certainty of success in your drug development programs. For more information, please visit the Certainty website.



