Drug-drug interactions (DDIs) occur when one drug affects the pharmacokinetics or pharmacodynamics of another drug. DDIs can result in reduced efficacy, increased toxicity, or unexpected side effects of the co-administered drugs. Therefore, biopharma teams should assess the potential for DDIs during drug development to ensure new drugs are safe, effective, and optimally dosed.
Mechanisms for this drug safety concern
One potential DDI mechanism is the inhibition of drug transporters, which are membrane transport proteins that uptake or efflux drugs and endogenous compounds across biological barriers (Figure 1). OATP1B (organic anion-transporting polypeptide 1B) is a family of hepatic uptake transporters involved in the clearance of many drugs, such as statins, angiotensin II receptor blockers, and anticancer agents. Inhibiting OATP1B1 and/or OATP1B3 can increase the plasma exposure of these drugs and potentially cause adverse reactions. Therefore, evaluating the inhibitory potential of new drugs on OATP1B1 or OATP1B3 is an essential part of DDI risk assessment.
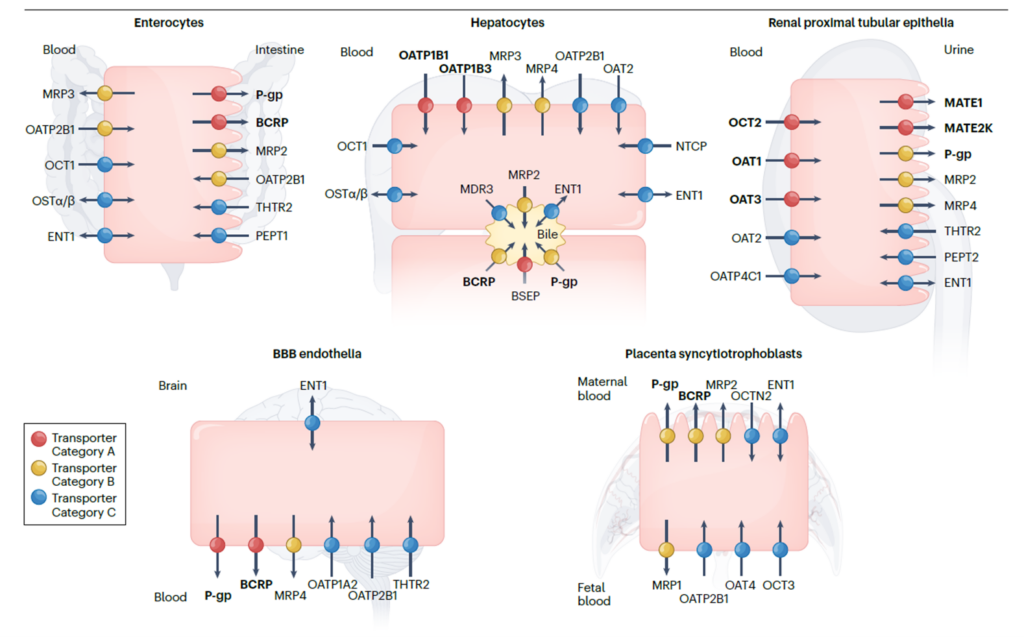
Figure 1. Clinically important uptake and efflux transporters in plasma. Galetin et al (2024). Membrane transporters in drug development and as determinants of precision medicine | Nature Reviews Drug Discovery
Coproporphyrin-1 (CP-1): An endogenous OATP1B biomarker for DDIs
Using the OATP1B biomarker, coproporphyrin-1 (CP-1), to guide DDI risk assessment was recently presented at the ISSX 2024 Workshop: Bringing Practical Applications of Endogenous Biomarkers for Drug Transporters a Step Closer. This concept was also described in the International Consortium for Innovation and Quality in Pharmaceutical Development (IQ) white paper published in Clinical Pharmacology & Therapeutics last December (Kikuchi et al, 2023).
CP-1 is a byproduct of heme synthesis in the liver or blood. It’s metabolically stable and predominantly eliminated via bile. CP-1 has been characterized in vitro as an OATP1B1 and 1B3 substrate, and this biomarker has robust clinical information (Kikuchi et al, 2023; Galetin et al, 2024), including a clear association of plasma CP-1 with the SLC01B1 genotype (e.g., genetically poor, decreased, normal or increased OATP1B1 function).
Is your drug an OATP1B inhibitor in vitro?
Did a DDI assessment from your in vitro transporter study suggest that your drug is a clinically relevant inhibitor of the OATP1B1 and/or OATP1B3 hepatic uptake transporters as per the upcoming ICH M12 guidance?
If so, know that the simple criterion used in the draft ICH M12 guidance tends to be conservative. Thus, your program may benefit from assessing an endogenous biomarker such as CP-1. The use of endogenous biomarkers was recognized in the draft ICH M12, which states that
“Alternatively, the inhibition potential of a drug can be evaluated using mechanistic static models, PBPK [physiologically-based pharmacokinetic] modeling, or endogenous biomarkers”.
The final guidance may include CP-1 recommendations based on scientific development in this field.
How to integrate CP1 into your clinical DDI risk assessment
The IQ working group presented a decision tree summarizing their recommendation for incorporating CP-1 in clinical OATP1B inhibition risk assessment (Figure 2).
CP-1 assessment early in clinical development (Phase 1 study in volunteers) will provide further information on how (if) the in vitro inhibition is expected to translate to the clinic.
With minimal intersubject variability in CP-1 baseline concentrations, measuring a single plasma pre-dose timepoint is sufficient, while the collection of a time course post-dose is recommended.
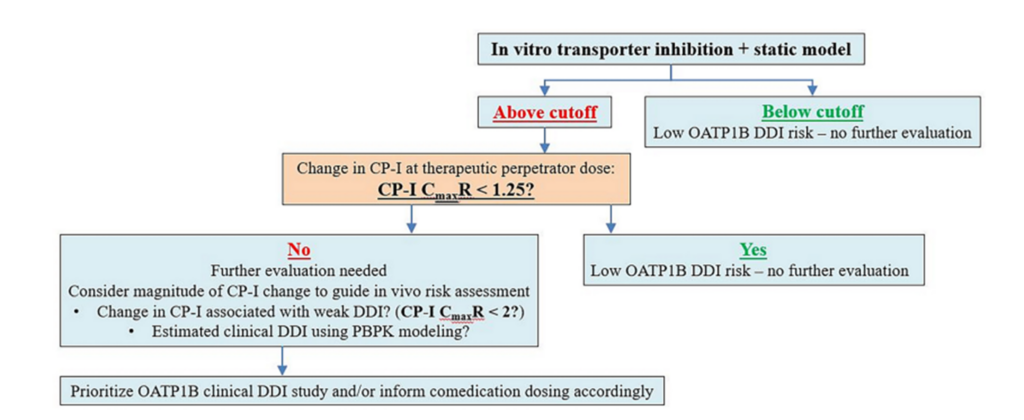
Figure 2. IQ OATP1B biomarker working group decision tree.
Other potential benefits of using CP-1 as biomarker of OATP1B
In conjunction with PBPK modeling approaches using the Simcyp Simulator software, CP-1 data can be used to determine a drug’s in vivo inhibition potency. Positive CP-1 results can be translated to a DDI assessment with OATP1B1/3 transported drugs, informing risk assessment for later-stage drug development, including the need for a dose adjustment of OATP1B substrates.
To learn how to use the Endogenous Biomarker Module in the Simcyp Simulator, please watch this video.
CP-1 data may also help elucidate clinical DDI mechanisms. As an example, the commonly used OATP1B clinical substrate rosuvastatin is also a substrate of the breast cancer resistance protein (BCRP). If a drug is a dual inhibitor of OATP1B and BCRP, a lack of change in CP-1 (which is not a BCRP substrate) will suggest that a change in rosuvastatin exposure following co-administration with this drug is driven by the intestinal efflux transporter BCRP and not OATP1B, having an impact on the drug label and potential for use as a co-medication with other OATP1B substrates.
Do you need support designing and interpreting in vitro and in vivo drug transporter studies? Our team at the Center of Excellence in Drug Interaction Science is ready to help!
To read about more examples of where we’ve helped clients streamline their drug safety assessment, read our DDI white paper.
References:
Draft ICH M12 guidance. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m12-drug-interaction-studies
Galetin et al (2024). Membrane transporters in drug development and as determinants of precision medicine | Nature Reviews Drug Discovery




