Rectal drug administration, though less common than oral methods, is crucial in delivering medication to patients who face challenges swallowing pills, are struggling with vomiting, or need targeted drug delivery. This route is particularly vital for children, the elderly, and patients with certain medical conditions. Additionally, rectal administration bypasses first-pass metabolism, ensuring the drug reaches its intended site of action effectively. A wide range of medications, from pain relievers to treatments for constipation, irritable bowel syndrome, nausea, and epilepsy, utilize the rectal route.
Despite these advantages, developing novel and generic rectal drug products faces significant challenges. Pre-clinical experimental methods for investigating candidate drug molecules and refining formulations are lacking. Furthermore, conducting clinical trials involving specific populations, such as children or the elderly, is difficult.
The Role of Model-Informed Drug Development (MIDD)
Model-informed drug development (MIDD) is a powerful tool to address these challenges. MIDD encompasses various computational methods that employ mathematical simulations to predict drug concentrations within the body. When well-developed and verified, MIDD methods can complement laboratory experiments and streamline drug development.
The Significance of Physiologically-Based Pharmacokinetic (PBPK) Models
One subset of MIDD tools is physiologically-based pharmacokinetic (PBPK) models. PBPK models simulate drug absorption and distribution within the body based on realistic mathematical descriptions of demographics, physiology, drug properties, and relevant biophysical and biochemical processes. These models are widely accepted in the pharmaceutical industry for predicting drug concentrations upon administration, assessing drug efficacy and safety, and establishing bioequivalence between generics and reference drugs.
The Need for a PBPK Model for Rectal Drug Absorption
The development of rectal drug products, including suppositories, can greatly benefit from a well-designed PBPK model. Such a model would fill gaps in pre-clinical research and facilitate clinical trials involving rectally administered drugs. At Certara, our Simcyp team recognized this need and embarked on a project to develop and test a model in the Simcyp PBPK Simulator for rectal drug absorption.
Introducing the “Mechanistic Rectal Absorption Model” (MechRAM)
Our research resulted in creating the “Mechanistic Rectal Absorption Model” or MechRAM. This model integrates physiological data concerning the human rectum from existing literature with mathematical descriptions of the physical processes governing drug uptake in the rectum when using suppositories. During the model’s development, we extensively reviewed scientific literature to determine relevant processes and assess the availability and quality of supporting data.
Model Verification and Validation
The MechRAM underwent rigorous testing and verification by simulating published clinical pharmacokinetic studies involving various rectally administered drugs. These studies used data independent of the information used to build the model, ensuring its predictive capabilities were reliable. In total, we tested and verified the MechRAM using clinical data from 10 different drugs, and in most cases, the model satisfactorily predicted the published clinical data. In instances where the model fell short, we thoroughly examined the reasons, as discussed in our paper.
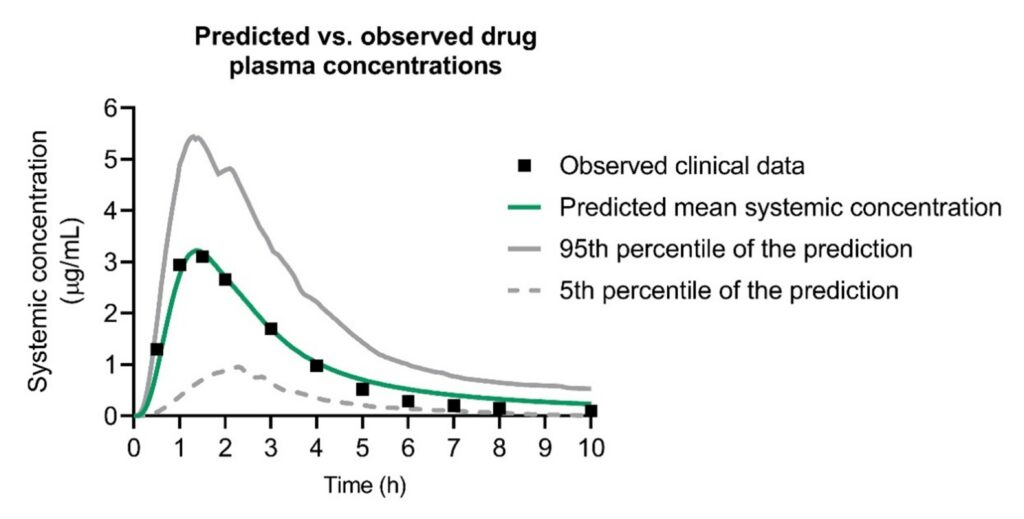
The Impact of MechRAM
The development of MechRAM is a significant step forward in rectally administered drug research. With suitable input data on the population and rectal drug product of interest, MechRAM reliably predicts drug concentration in the bloodstream after rectal administration. We anticipate it will be an invaluable tool for pharmaceutical companies interested in rectal drug product development.
Closing the Gaps
Additionally, our case studies highlighted gaps in our understanding of the physics of drug uptake in the rectum. The rectal route remains understudied compared to other drug administration routes. Our paper aims to raise awareness within the pharmaceutical sciences community and encourage more in-depth mechanistic research into rectal drug administration.
Implications for Regulatory Bodies
We anticipate that MechRAM will also garner the attention of regulatory bodies, including the U.S. Food and Drug Administration (FDA). The FDA actively champions research into both experimental and computational methods aimed at predicting drug concentrations within the body. This research plays a pivotal role in establishing bioequivalence between generic drugs and their reference counterparts. While research in the realm of rectal drug administration is in its infancy, MechRAM boldly pushes the boundaries, setting a new gold standard for rectal drug product development.
In conclusion, the development and validation of MechRAM signify a momentous stride forward in the field of rectal drug administration. This groundbreaking PBPK model not only streamlines drug development processes but also serves as a catalyst for further exploration in this relatively uncharted domain of pharmaceutical science. The potential benefits for patients and the pharmaceutical industry are substantial, and we eagerly anticipate witnessing MechRAM’s profound impact on future drug developments and regulatory decisions. For more comprehensive insights into how our pioneering PBPK model accurately simulates local and systemic drug concentrations following rectal administration of various drug formulations in human subjects, we invite you to read our scientific publication.




