July 29, 2024
(medical devices)
(associated persons)
(pharmacogenomics, pharmacogenetics)

Get up to speed on CDISC standards
CDISC standards can be complex, and there’s a lot to consider in terms of compliance. That’s why we’ve put together a complete guide to CDISC standards – to help steer you down the right path towards compliant submissions. So download your free step-by-step guide and see how to overcome common challenges become an expert in managing CDISC standards.

Customer Success Manager
Gilbert joined Formedix, now part of Certara, nearly ten years ago as a technical writer. The system knowledge he gained from content development, together with his existing customer service skills, marked him out for transition to the Professional Services (PS) team.
Gilbert has worked with the PS team for over four years, providing both CDISC-based training and software training, as well as support and consultancy services to Pharmaceutical, Biotechnology and Clinical Research Organizations. He helps organizations build studies faster and to a higher quality by making their clinical trial design and regulatory submissions far more efficient.
Today, as Customer Success Manager, Gilbert’s focus is to ensure customers maximize the benefits they can achieve by overcoming their challenges and achieving their goals.
Schedule a consultation with Certara
FAQs
Why are CDISC standards required by the FDA?
The FDA mandates CDISC standards because they allow for consistent data formats across submissions. This reduces errors, accelerates reviews, and ensures datasets are comparable across studies.
What CDISC standards are required for clinical trial submissions?
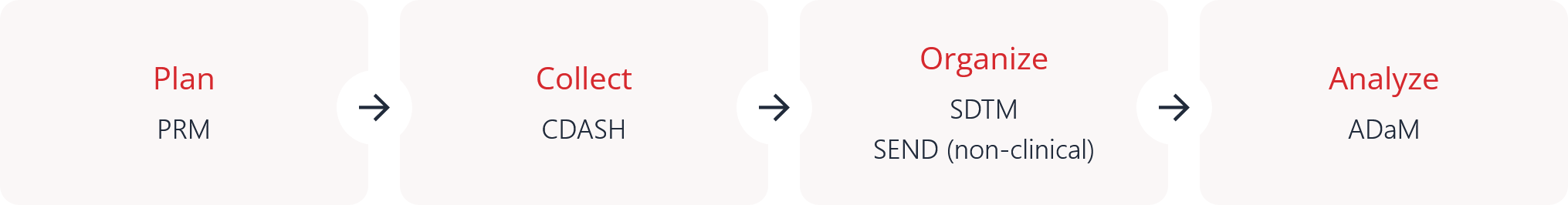
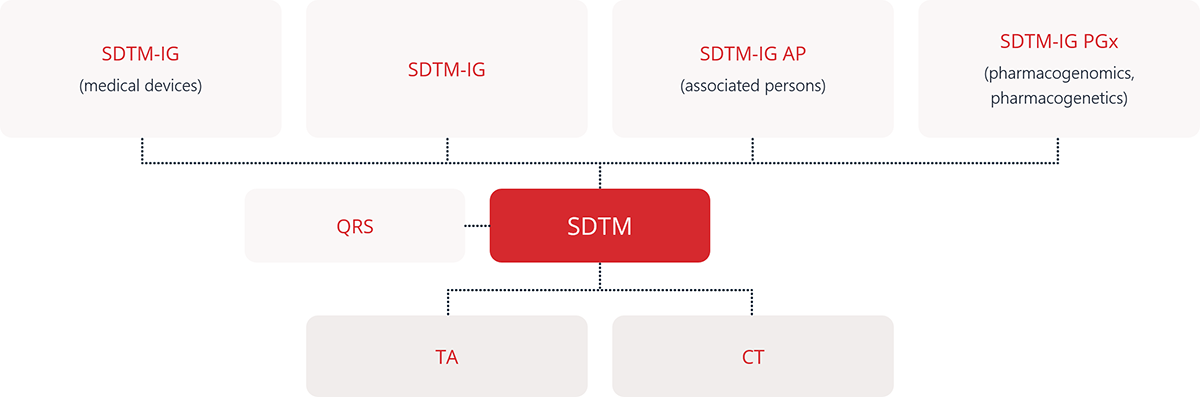
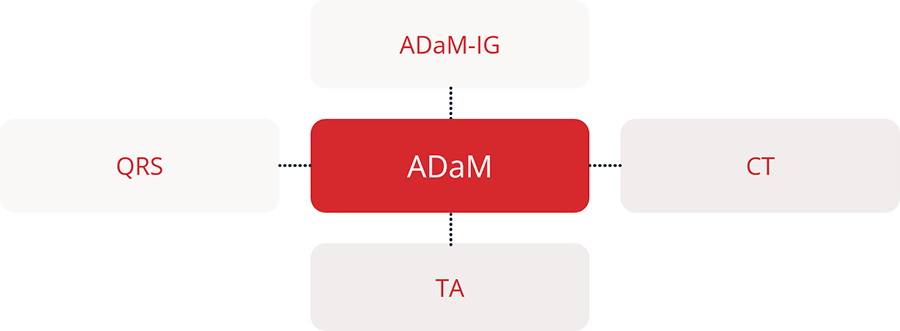
Regulatory agencies such as the FDA and PMDA require key CDISC standards for clinical trial data submissions to ensure consistency, traceability, and transparency. The primary standards include SDTM for organizing collected data, ADaM for analysis-ready datasets, and Define-XML for describing dataset structure and metadata. In addition, Controlled Terminology ensures consistent use of standard terms, while SEND (Standard for Exchange of Nonclinical Data) applies to nonclinical studies. Learn more.
How do CDISC standards benefit sponsors and CROs?
CDISC standards reduce the cost and time of data preparation, minimize rework, and ensure datasets are submission-ready, improving collaboration between sponsors, CROs, and regulators.