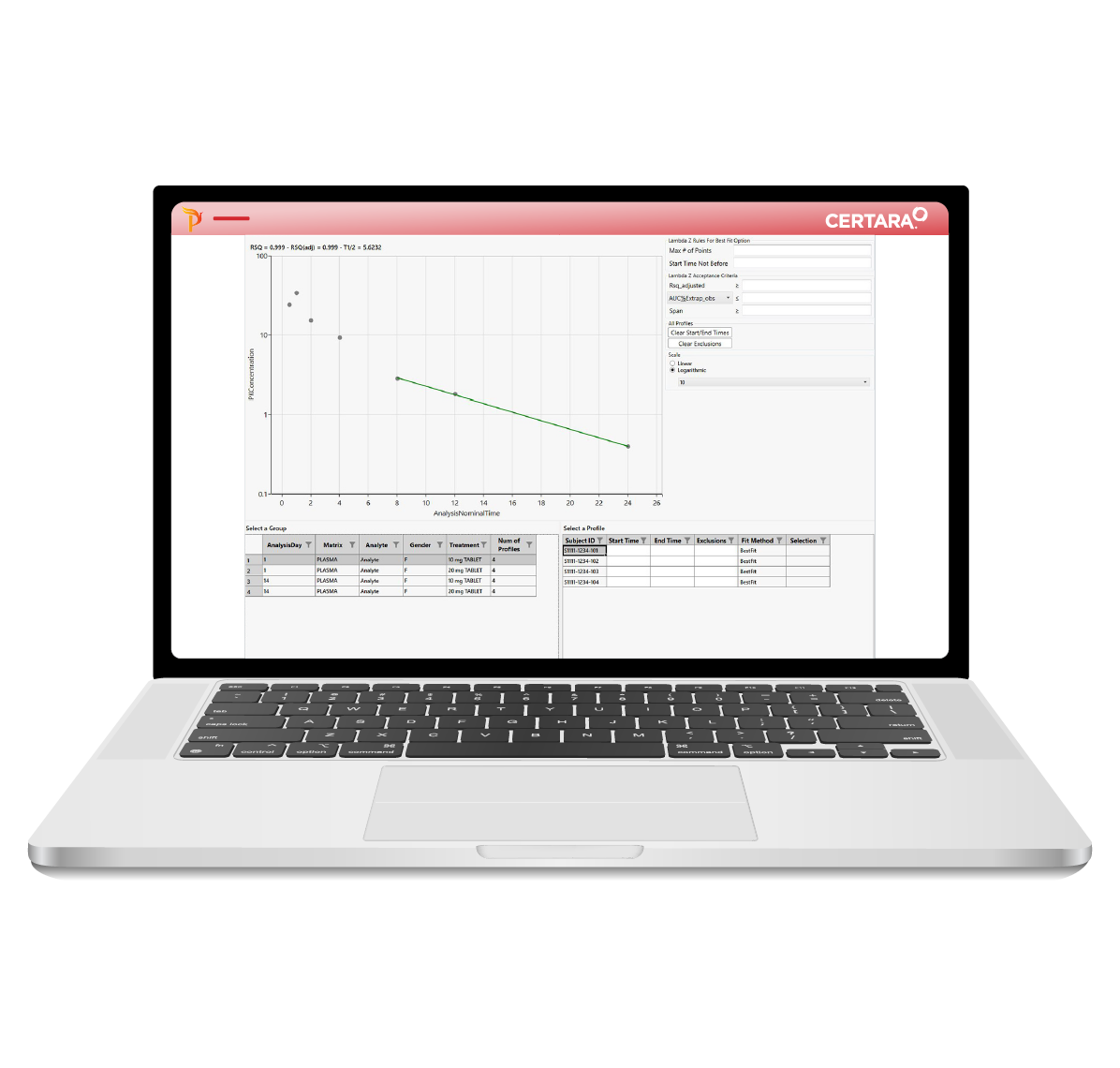
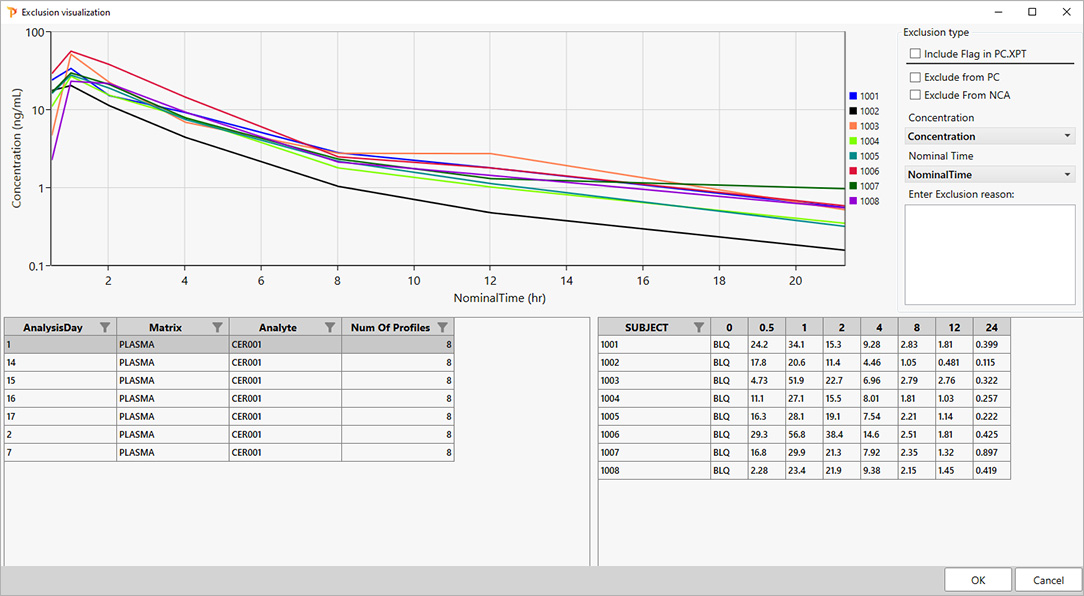
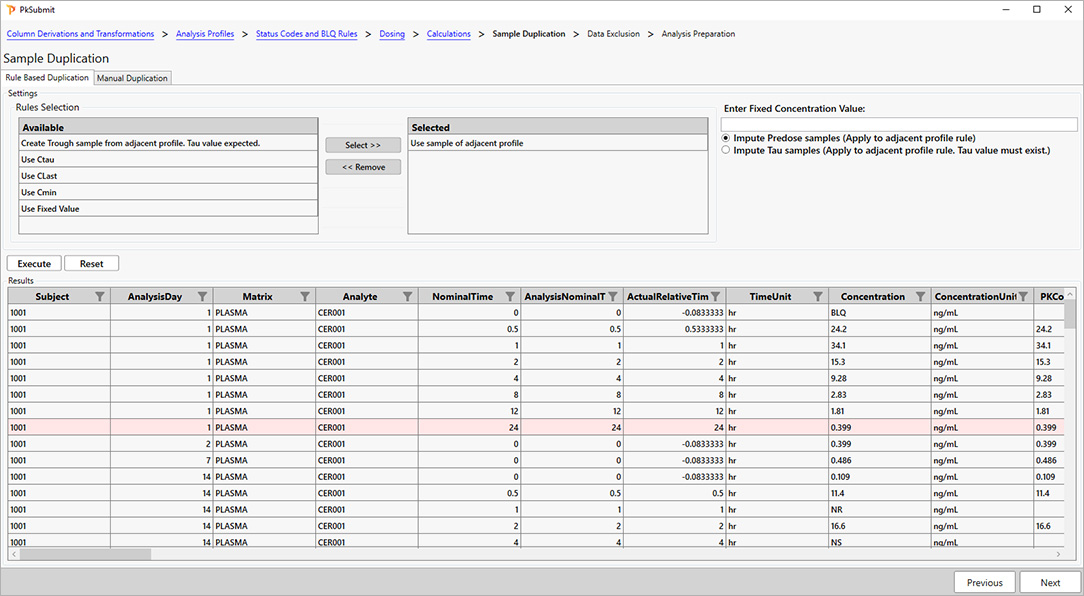
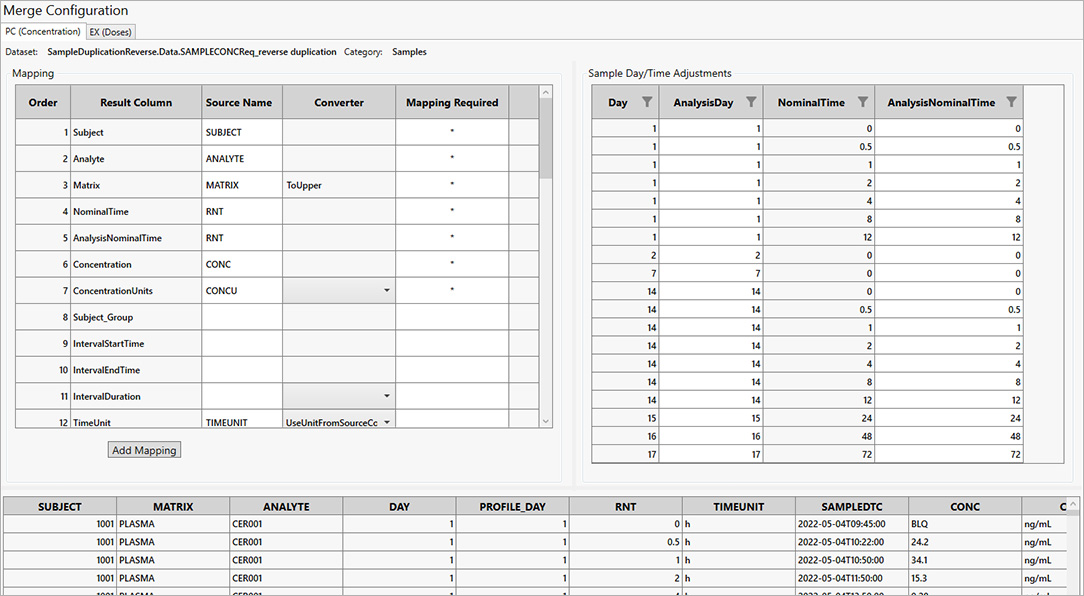
For PK scientists, speed and accuracy are essential at every stage of the drug development lifecycle. Certara PK Submit® is a powerful plug-in for Phoenix®, designed to streamline non-compartmental analysis (NCA) and generate validated PK-related CDISC submission datasets.
By automating critical steps and offering a guided, intuitive user interface, PK Submit reduces the time and complexity involved in preparing and analyzing PK datasets. This innovative solution enables scientists to focus on extracting valuable insights from study data while ensuring compliance with regulatory standards.