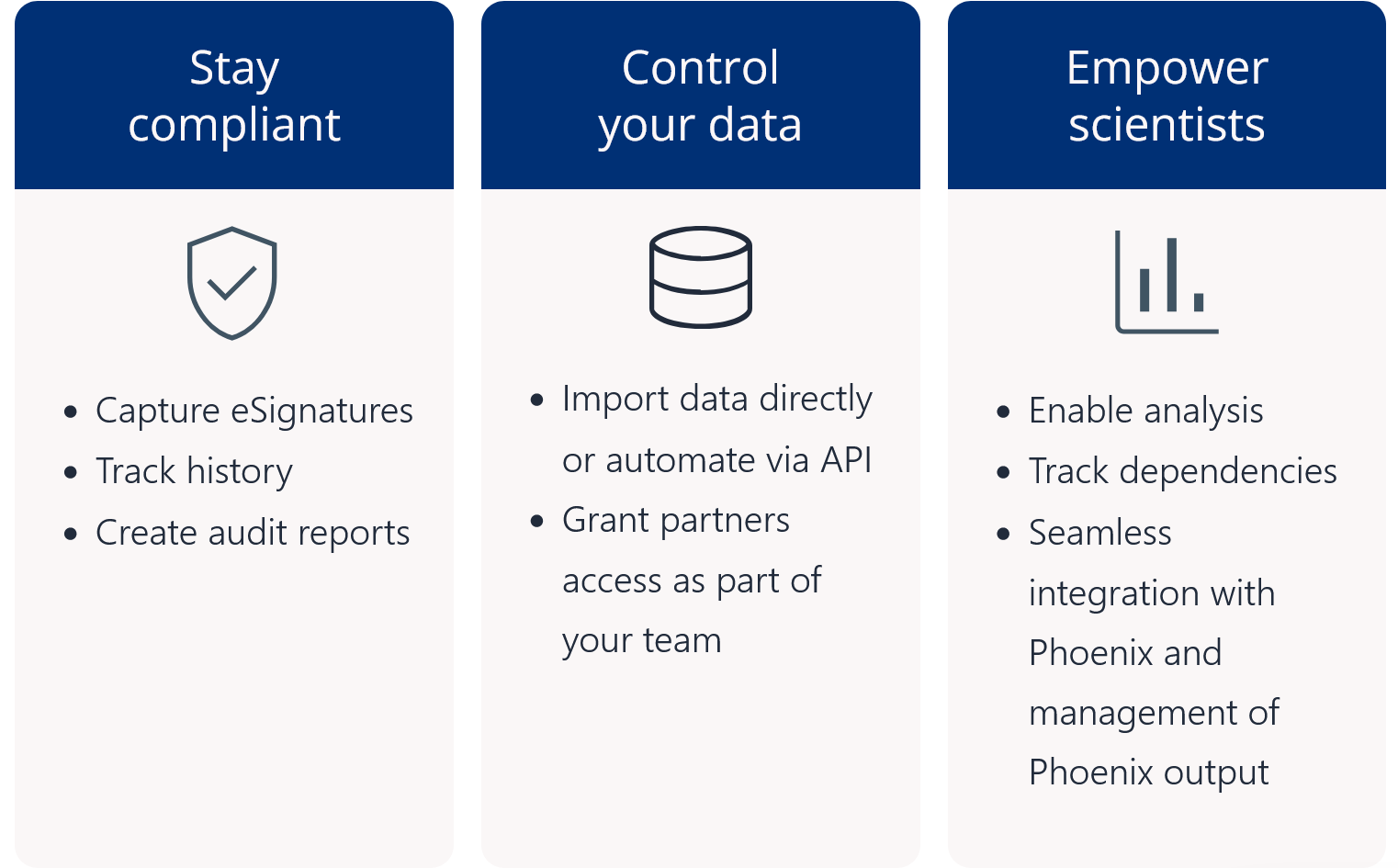
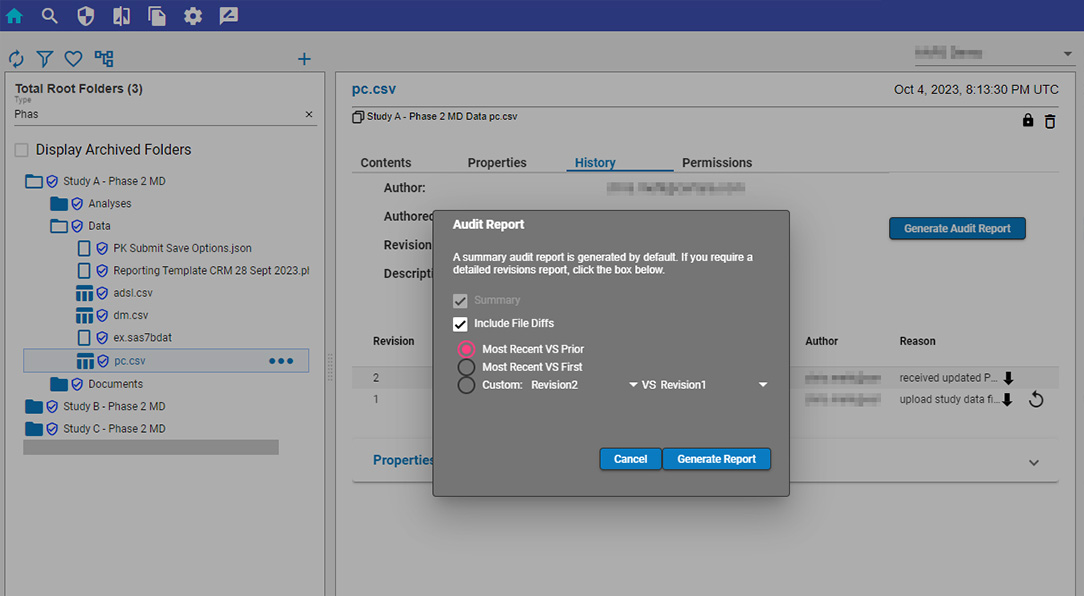
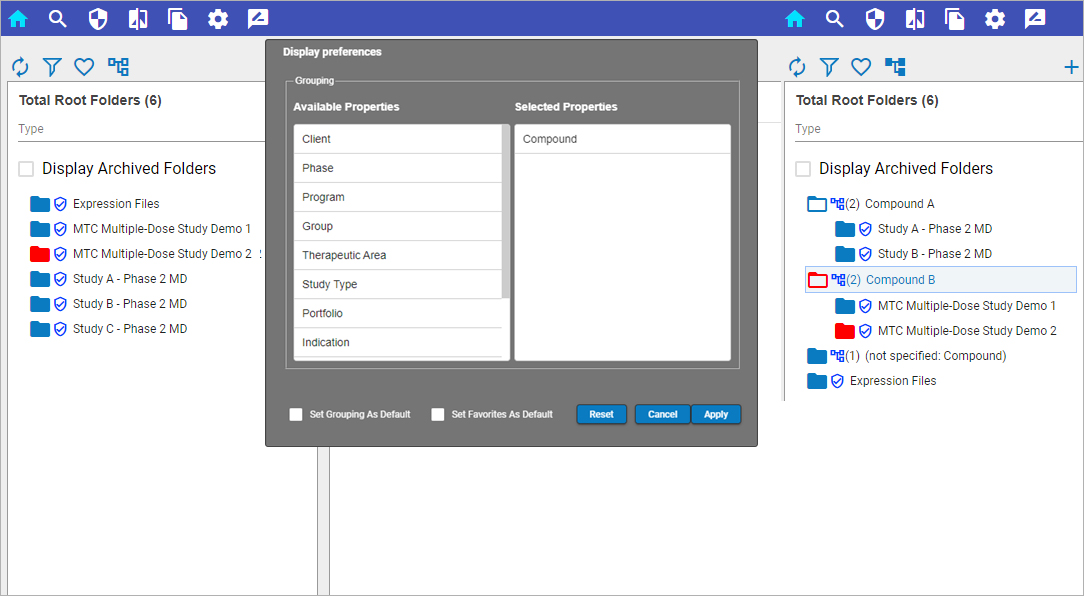
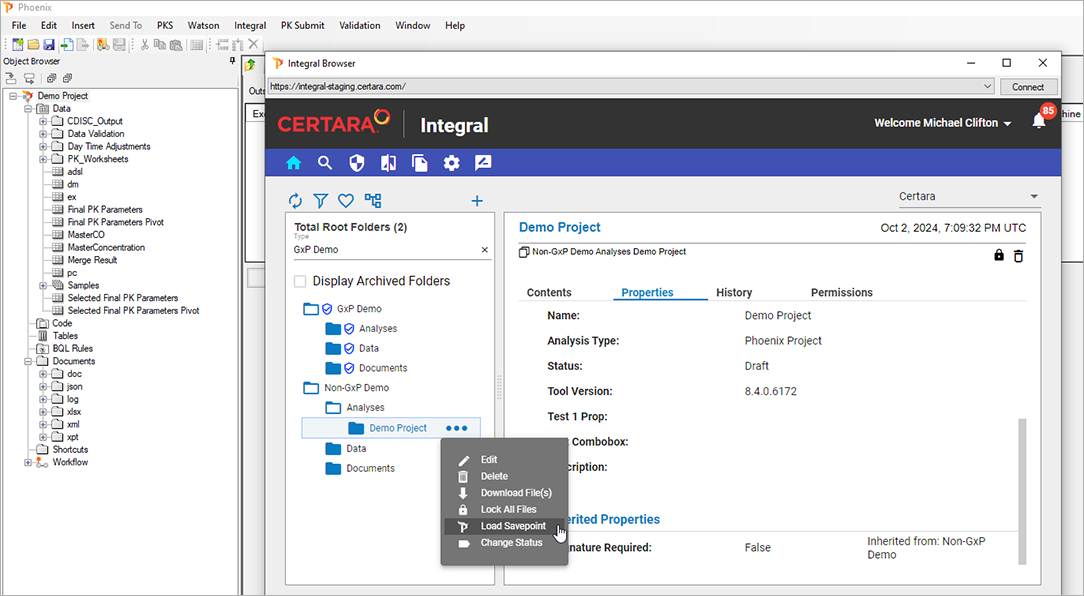
Pharmacometric teams face many data-related productivity challenges. For example, version control problems and data silos may create weeks of quality control and aggregation work before scientists can begin modeling and analysis. Those lucky enough to have a single source of data often still struggle to find, access, and work with the data in their analysis and modeling tools. Once found, they may need to manually upload delimited files and then repeat the process all over again each time the data is updated. Too often, these manual processes lead to costly errors and delays.
Certara Integral® is a purpose-built data and model repository for the pharma and life sciences industry. Integral provides complete functionality for PK scientists to manage the data lifecycles for both GxP and non-GxP studies and helps facilitate their analysis work and drive efficiency.