The time and financial pressures drug development teams face is immense, and when factoring in failures and capital expenditures, the cost to develop a new drug increases fivefold. Improve your odds of success with expertly curated clinical trial outcomes data and visualizations. Certara’s analysis-ready CODEX application provides a cost-effective solution, capturing key safety and efficacy endpoints, and facilitating comparison of treatments even in the absence of head-to-head trials. CODEX will empower your team to design superior trials, accurately predict later-stage outcomes, and reduce the prevalent >90% failure rate dominating current drug development programs.
CODEX
Analyze, visualize and compare clinical trial outcomes data with the AI-powered CODEX Clinical Outcomes Databases
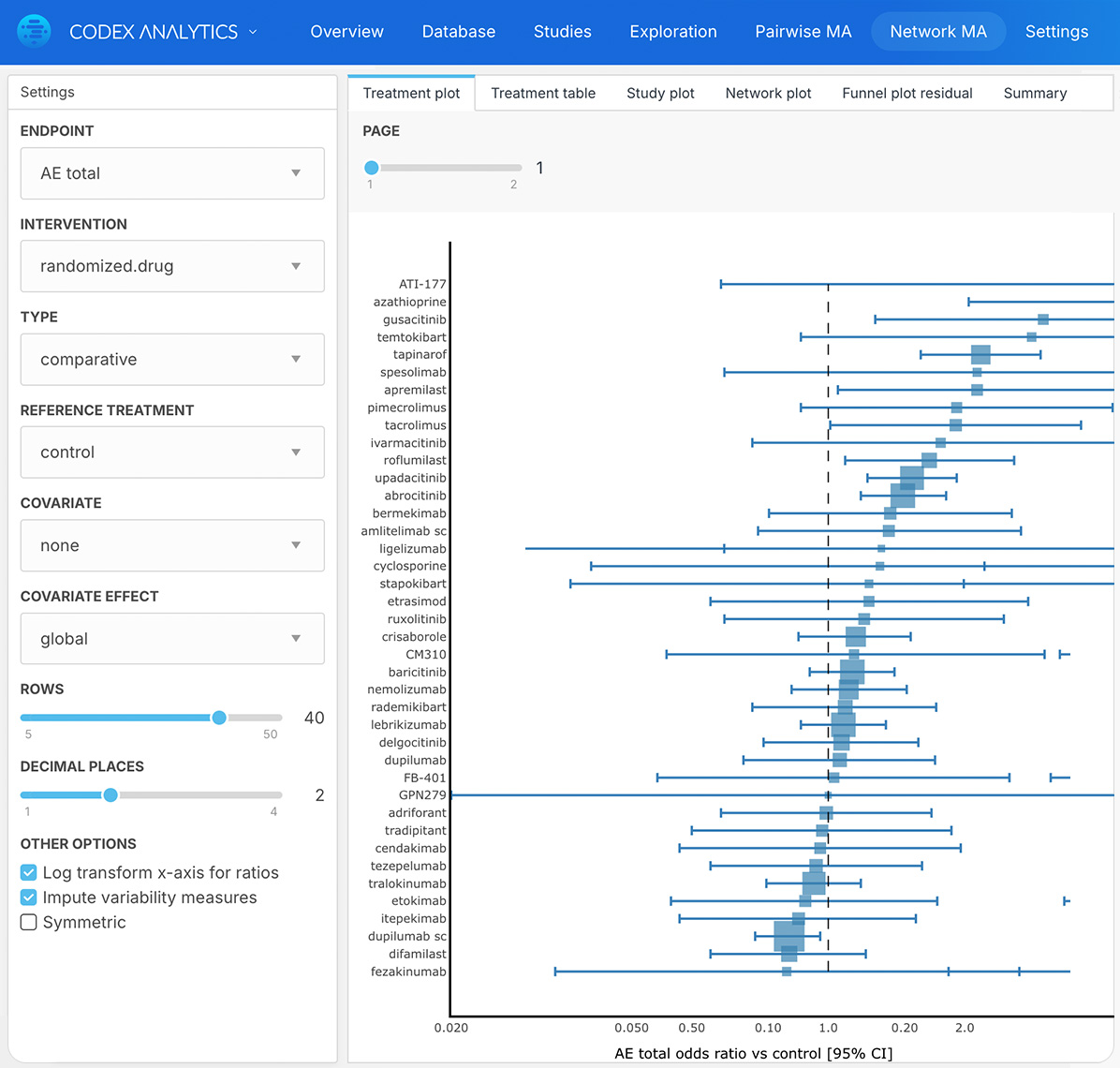
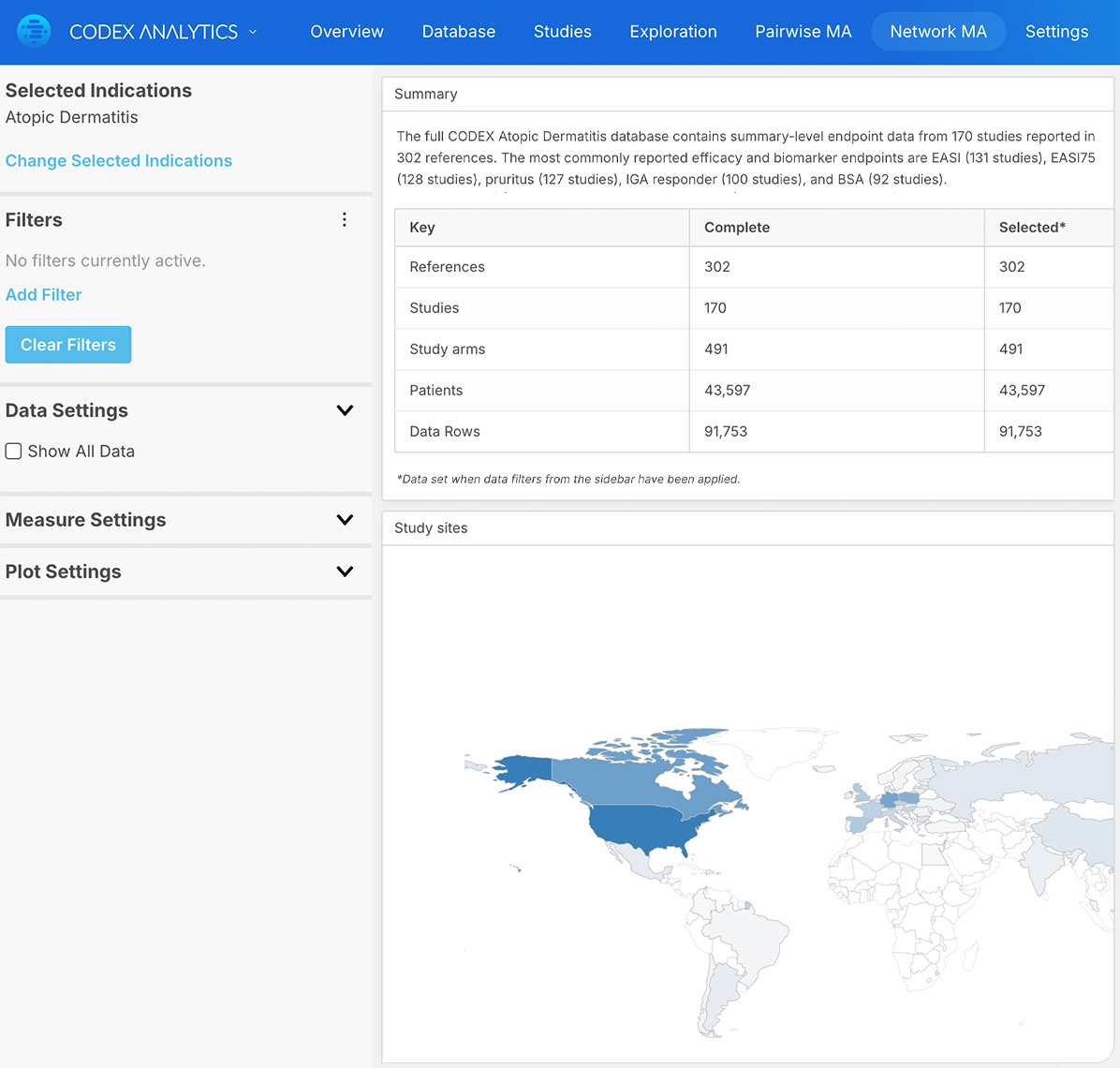
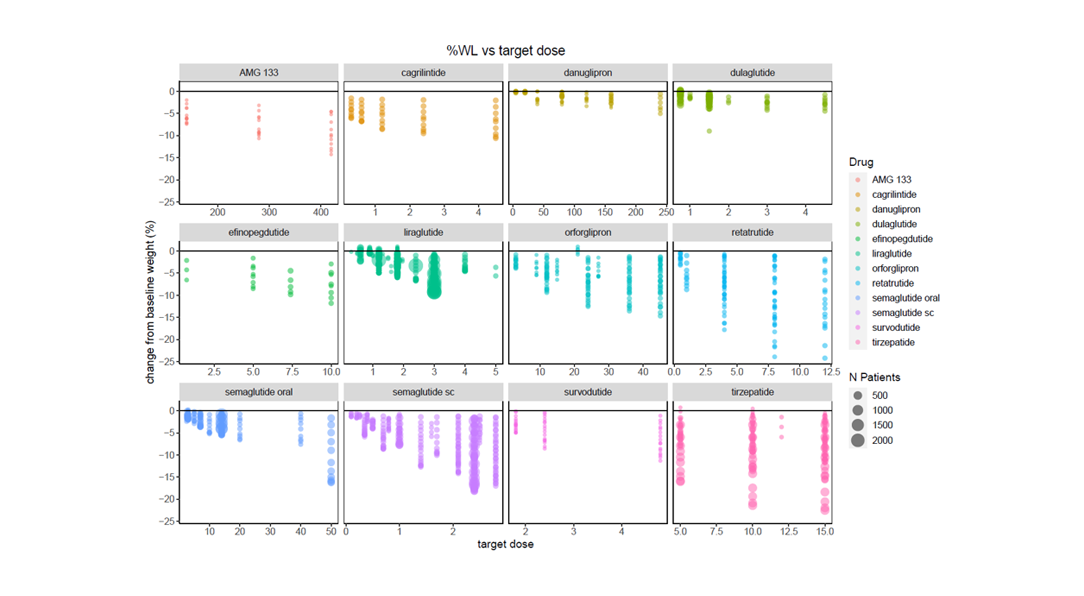
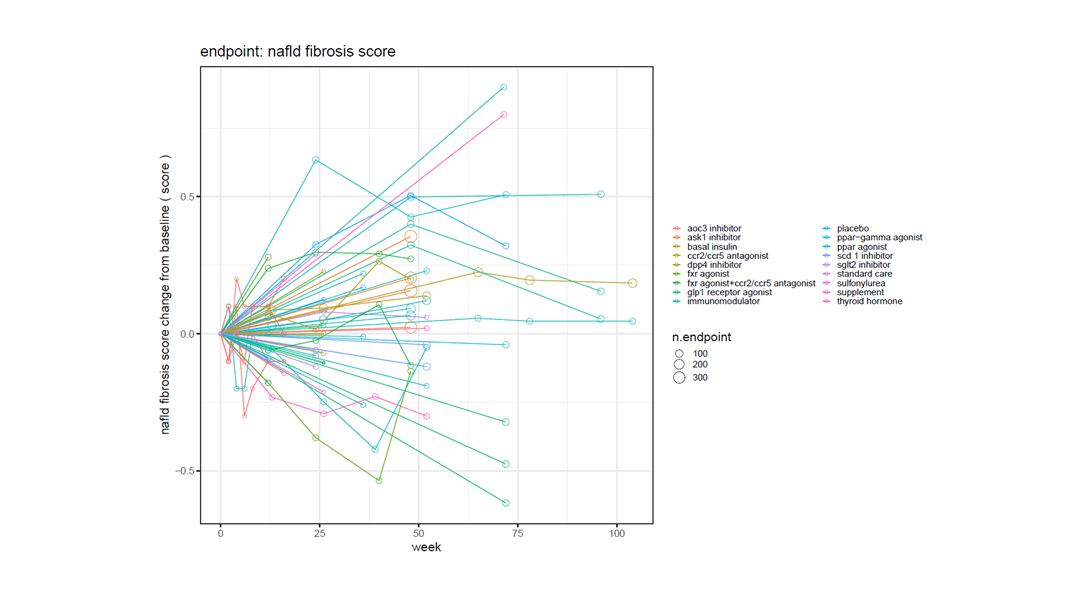
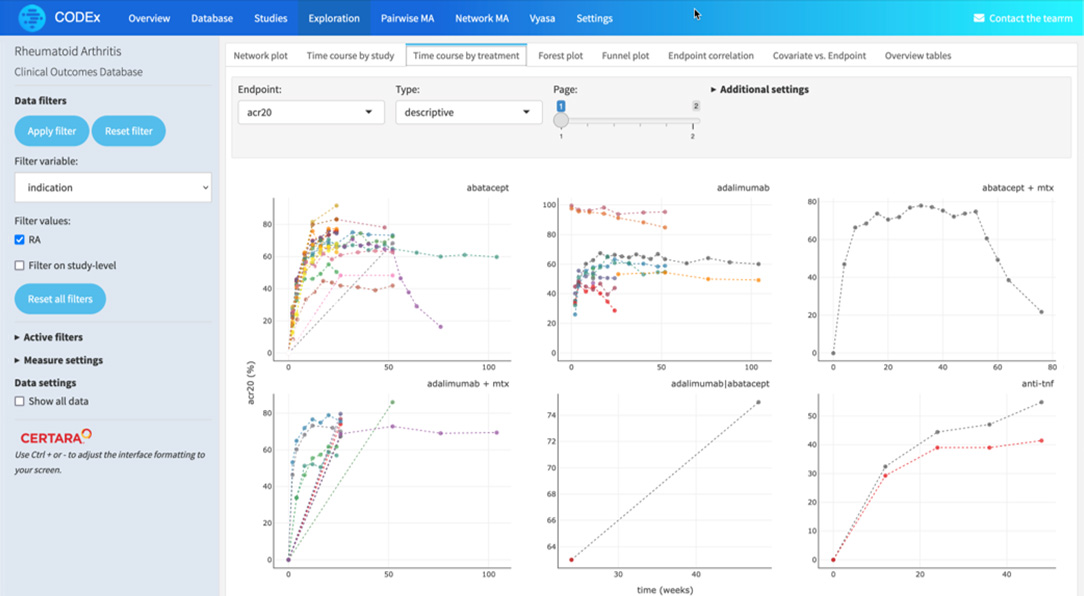
Apply filters for endpoints, dosage, study type and population for rich and relevant insights
Certara’s Clinical Outcomes Database (CODEX) analytics provide unparalleled insights into marketed drugs and drugs in development. By combining expertly curated clinical trial outcomes databases with powerful analytics, CODEX provides the insights clinical development teams need to improve study design, assess the competitive landscape and anticipate outcomes.
Do more with clinical outcomes data
Most popular CODEX indication databases
Certara.AI and CODEX
Certara leads the industry in advancing AI-powered solutions for clinical trial optimization. Seamlessly integrate your internal data for meta-analysis tasks and utilize our AI-powered document extraction tool to effortlessly craft custom datasets tailored to your unique requirements.
Transforming trial success: AI’s role in clinical trial meta analysis
In this webinar you’ll learn how meta-analysis helps researchers reach conclusions about efficacy based on clinical trial results, and how to translate those learnings into real-time strategies that improve study outcomes.

Related resources
View all
Your data is safe with CODEX
Certara adheres to the highest standards of data security and compliance, ensuring your information is protected.
Schedule a demo
Discover how Certara’s Clinical Outcomes Databases can transform your clinical trial strategies. Schedule a demo today to experience the power of AI-driven insights.
FAQs
What therapeutic areas are covered in the Clinical Outcomes Databases?
The databases span 65+ indications and eight therapeutic areas including immunology, oncology, metabolic disorders, and CNS conditions.
How does CODEX integrate internal and external data?
CODEX allows seamless integration of internal data with external public data sources for comprehensive meta-analysis.
Is CODEX user-friendly for non-technical users?
Yes, CODEX features a no-code analytics tool and an intuitive interface for easy data exploration and visualization.