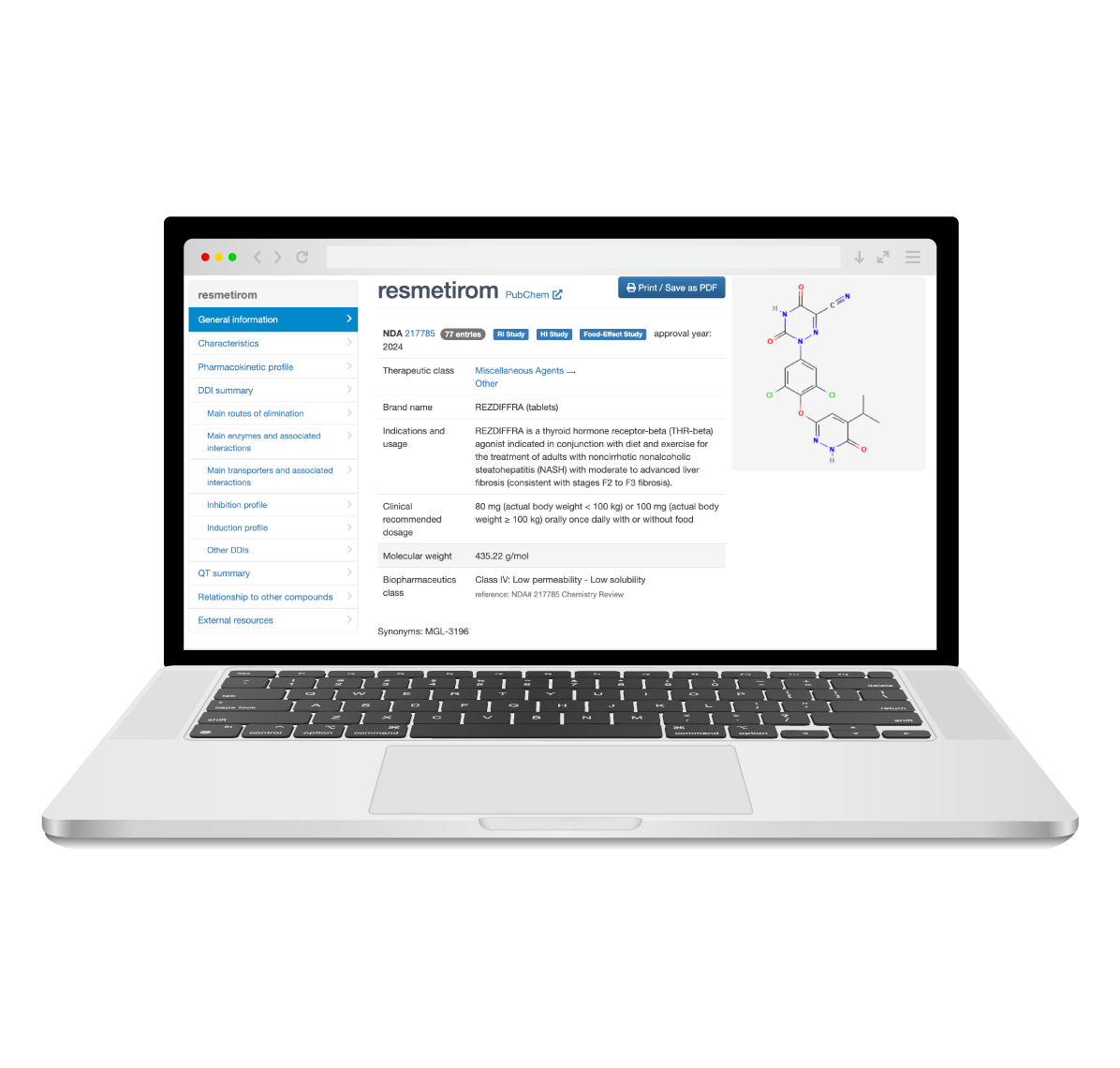
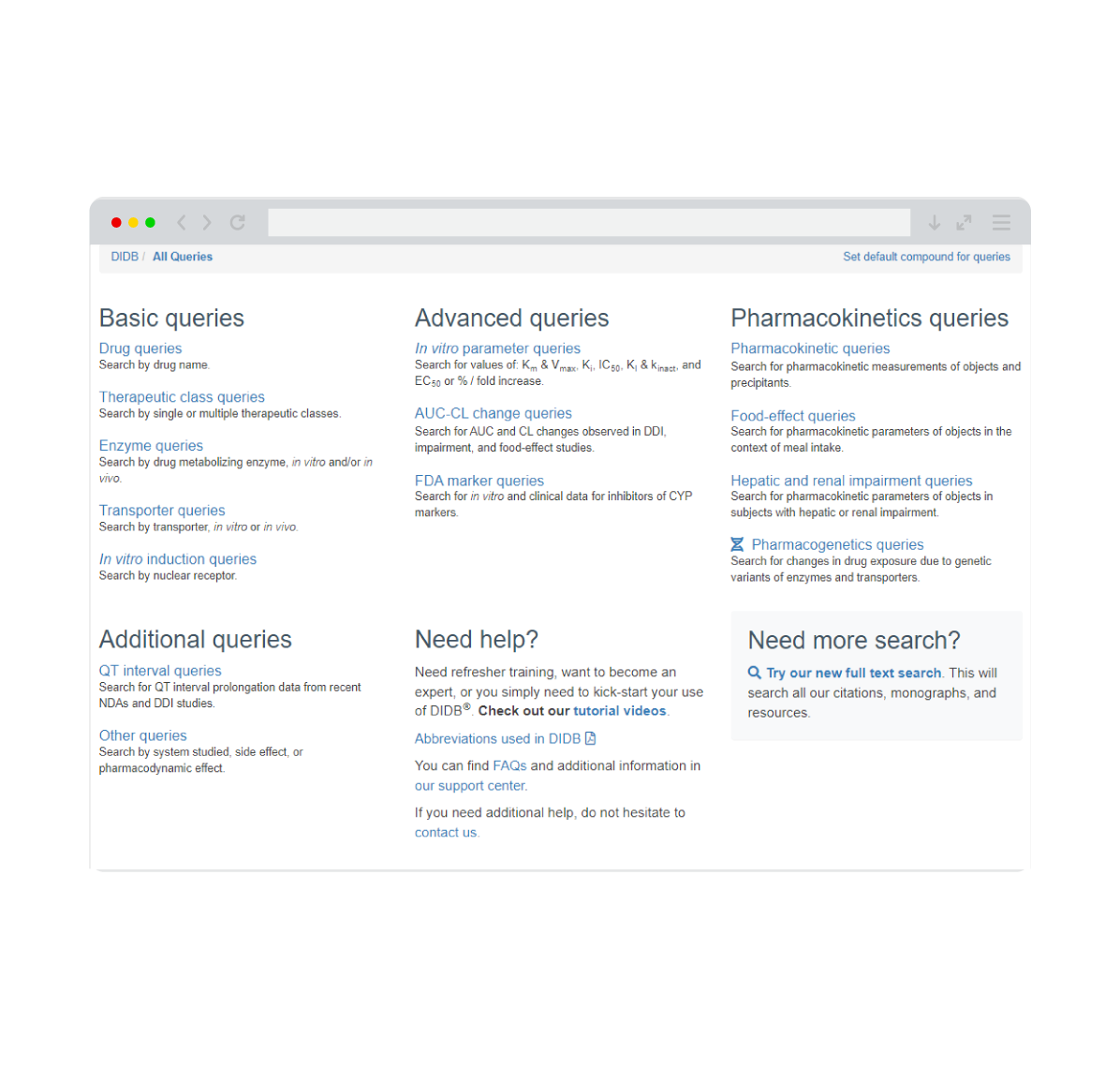
The Drug Interaction Database (DIDB®) is the industry’s most comprehensive resource for evaluating drug interactions. Trusted by over 200 pharmaceutical and biotech companies, as well as regulatory agencies, DIDB® provides unparalleled access to qualitative and quantitative human in vitro and clinical data.
By incorporating information on extrinsic and intrinsic factors such as co-medications, excipients, food products, natural products, organ impairment, and genetics, DIDB® enables informed decision-making and supports the development of safer, more effective therapies.