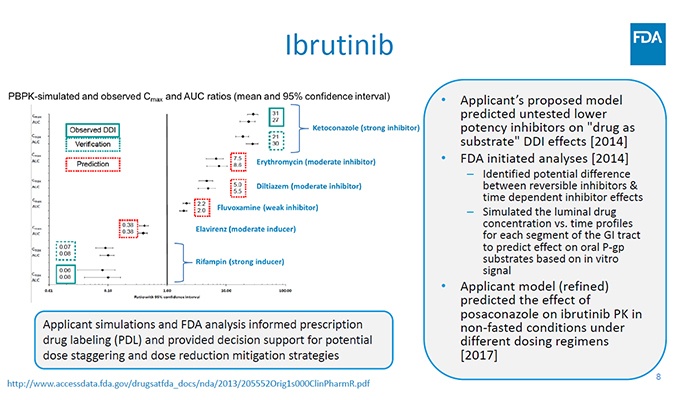
Pharmacylics and J&J sought to bring ibrutinib, its new tyrosine kinase inhibitor therapy targeting rare B-cell malignancies to market. The company leveraged FDA’s accelerated approval program; it was one of the first to be awarded breakthrough status by the agency. Ibrutinib is susceptible to interactions with a strong inhibitor and inducer of CYP3A4 enzymes, thus posing potential drug-drug interactions (DDI). Additionally, a dose optimization approach was needed to adjust the regimen for cancer populations.
Models built in the Simcyp Simulator using in vitro data were validated using clinical data on the observed effects of both a strong CYP3A4 inhibitor and a strong inducer on ibrutinib exposure. Simulations then predicted the effects of a moderate CYP3A4 inducer and other CYP3A4 inhibitors (strong, moderate, and weak) on ibrutinib exposure, as well as investigating the impact of dose staggering and dose adjustment. The final drug label included 24 individual claims for untested DDI scenarios (without the need for clinical trials) and provided a dose optimization strategy aligned to individuals with different metabolic profiles.
Contact us