Per US FDA’s recent paper on the topic, Physiologically based Pharmacokinetic (PBPK) modeling & simulation impact on drug approval has grown significantly over the past years, with about 45% percentage of new drug approvals including PBPK analyses in 2019 [1].
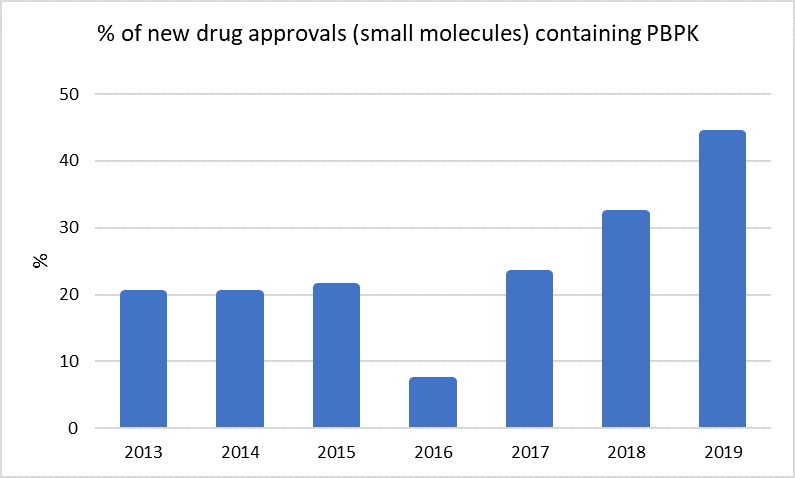
Another paper showed a rise of more than 280% in pharmaceutical applications of PBPK M&S publications over the last 10 years [2].
With increasing acceptance by global regulators in the use of PBPK for drug-drug interaction (DDI) risk predictions and for biowaivers, PBPK has now become a core part of drug development programs. Areas of application are wide ranging— from the readily acknowledged enzyme-mediated DDI and PBPK modeling to predict first-in human pharmacokinetics (FIH PK)— to transporter-mediated DDI, hepatic impairment, renal impairment, absorption, acid-reducing agent-mediated DDI, food effects, pediatrics, pharmacogenomics, and the prediction of drug concentration in specific tissues/organs, such as liver.
Adopted by 12 global regulatory agencies including the US FDA, Japan’s PMDA, UK’s MHRA and others, the Simcyp Simulator has been used to inform scores of drugs, replacing the need for clinical trials, and providing prescribing information for approximately 250 label claims. The Simcyp Simulator continues to achieve industry and regulatory milestones, establishing itself as the leading global PBPK technology platform.
Simcyp is available via licensing or consultancy and if you are not already a Simcyp user, here are Top 10 reasons to join the ever growing Simcyp family…
Accelerate
1. The Simcyp PBPK Simulator enables you to accelerate your clinical development by avoiding and/or replacing clinical trials with PBPK modeling.
2. Quickly perform early PK first-in-human dose assessments to triage potential compounds with best chance of success.
3. Test alternative formulations in silico with the Simcyp Simulator.
Advise
4. Advise clinical trial design to reduce the study size by refining patient cohorts via PBPK.
5. Strengthen your NDA/BLA or regulatory filing package by answering ‘what if’ questions.
6. Use Simcyp Simulator to make key drug development decisions from pre-IND to post-marketing.
7. Bridge your data to untested populations, such as pediatrics and organ-impaired patients.
Accredited & Accepted
8. Leverage hundreds of peer-reviewed papers and unique application case stories authored by Simcyp staff, industry partners, academic users, and regulators.
9. Trusted and used by >10 global regulatory agencies, including the US FDA, for drug label review and approvals.
10. The Simcyp Simulator is proven for attaining biowaivers and demonstrating virtual bioequivalence (VBE) in complex generics.
Most importantly, Simcyp Simulator will allow you to bring drugs to patients faster and improve ROI through smarter decision-making, improved compound selection and trial avoidance/reduction.
Join the 36 Simcyp Consortium members, 130+ Biotech companies, 100+ Academic partners and >10 Global Regulatory agencies.
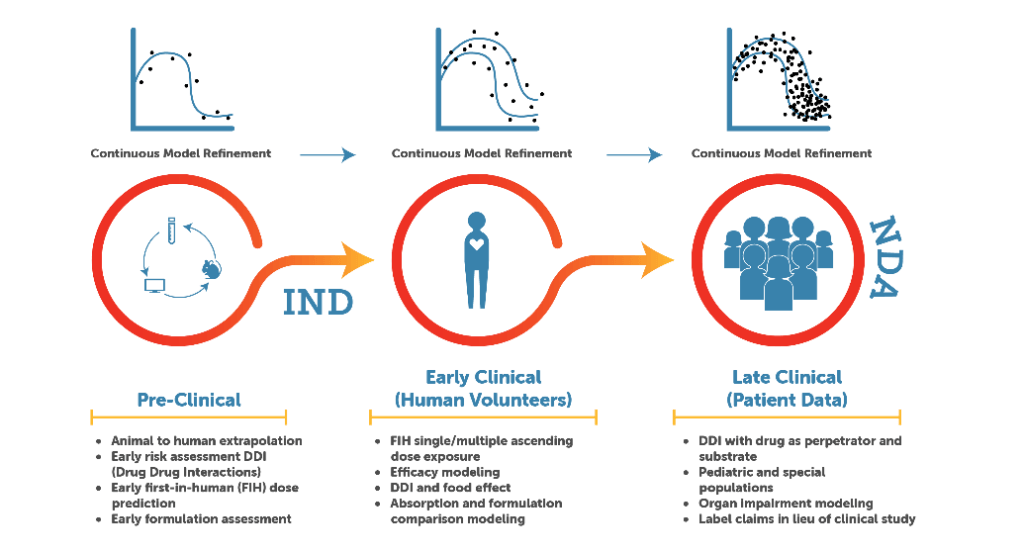
The Simcyp Simulator provides benefits across the development cycle
For more information, we suggest you download Certara’s whitepapers on Simcyp PBPK’s impact on DDI or Simcyp PBPK’s impact on pediatric drug development, or contact us at [email protected].
- Zhang, X., et al., Application of PBPK Modeling and Simulation for Regulatory Decision Making and Its Impact on US Prescribing Information: An Update on the 2018-2019 Submissions to the US FDA’s Office of Clinical Pharmacology. J Clin Pharmacol, 2020. 60 Suppl 1: p. S160-S178.
- El-Khateeb, E., et al., Physiological-based pharmacokinetic modeling trends in pharmaceutical drug development over the last 20-years; in-depth analysis of applications, organizations, and platforms. Biopharm Drug Dispos, 2021. 42(4): p. 107-117.


