February 14, 2025
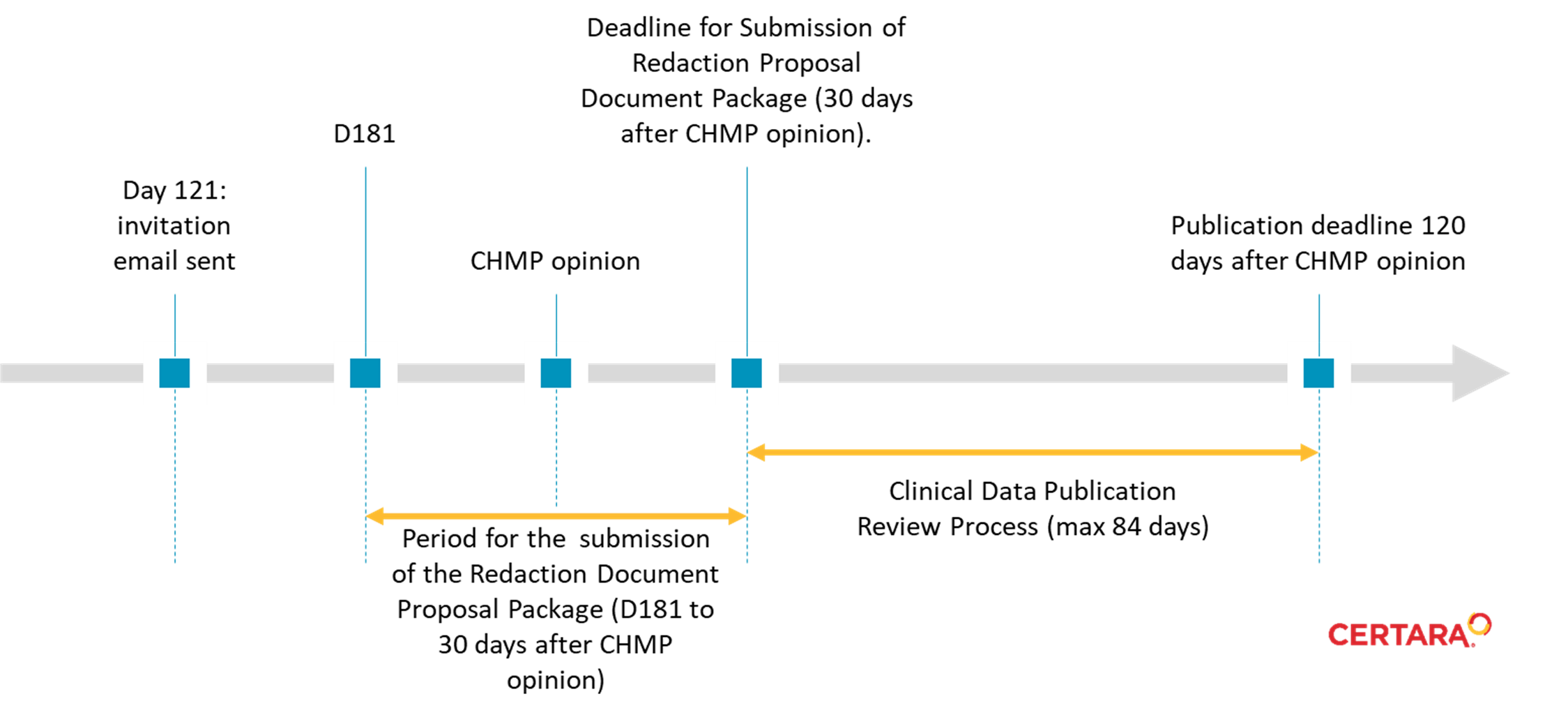
Figure 1. Clinical data publication timeline for initial MAAs
Clinical Trial Data Sharing Under EMA Policy 0070
Read our white paper to learn how to save time and resources while disclosing clinical trial data.
This blog was originally published on May 23, 2023, and was updated on February 14, 2025.
References
EMA Clinical data publication. Accessed February 5, 2025.
Learn more about Transparency & Disclosure
Certara’s clinical trial transparency and disclosure services enable biotech and pharmaceutical companies to meet global regulatory requirements while fostering trust and engagement.


Senior Transparency Specialist, Certara
Anaya Rehman has more than 10 years of experience in healthcare, academic research, and the pharma industry. She provides technical leadership and subject matter expertise for the clinical trial transparency and disclosure team at Certara. She works with clinical trial sponsors to meet the stringent requirements of regulations such as EMA Policy 0070, Health Canada Public Release of Clinical Information and European Union Clinical Trial Regulation 536/2014.

Senior Director, Transparency and Disclosure, Certara
Evan Richardson began his career in the pharmaceutical industry 18 years ago, and today he is the Senior Director of Transparency and Disclosure services at Certara. His experience includes drugs, biologics, and medical devices and he has worked for both service providers and in industry across organizations of all sizes. Evan has worn many hats throughout his career, including roles in regulatory affairs, regulatory operations, quality management, and project management. In his current role, Evan leads a global team of subject matter experts supporting Certara’s clients in meeting their regulatory requirements for the disclosure of clinical data.
Contact us





