Many drug developers have products that they want to submit for marketing approval in several regions like the US (NDA/BLA), Europe (MAA (Marketing Authorization Applications)), Canada (NDS (New Drug Submissions)), and elsewhere. But developing each submission independently can waste a lot of time and money. Fortunately, there are ways to maximize the reusability of your marketing applications to streamline gaining approval from multiple health authorities.
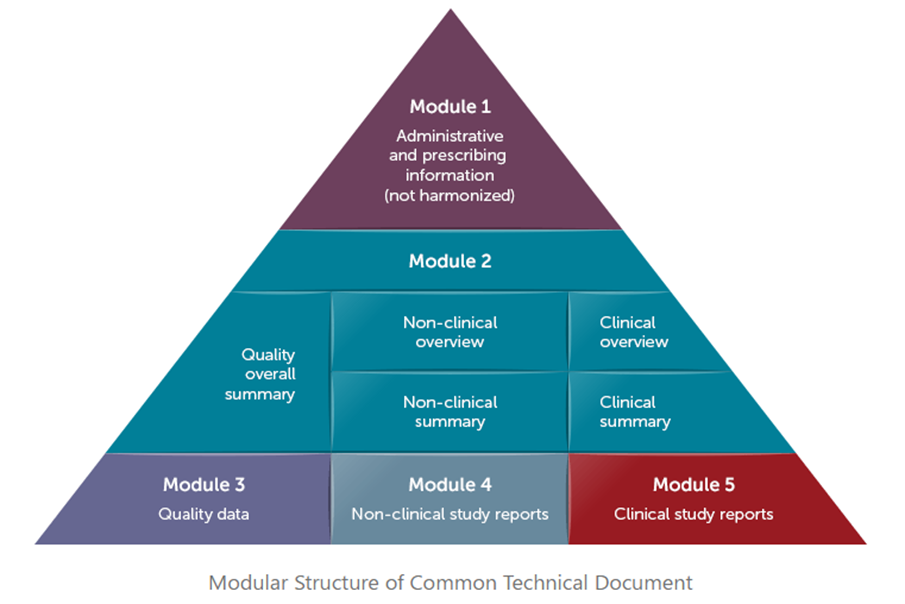
Preparing your submissions under the Common Technical Document (CTD) is the beginning of harmonizing your submissions. But it’s not as straight-forward as you might think. There are some key differences in submission requirements between regions that you need to consider, even in the sections that are “common.” Most people will tell you that Module 1 is region-specific, and Modules 2-5 are common. That’s not exactly the case.
What areas are not so common?
- Module 1 (Administrative) is region-specific so there is little, if any, straightforward reuse. (Though some content can be re-tooled to meet the requirements of a different region.)
- Module 2 (Summaries): Some of the summary documents may be able to be reused, but experience has shown us that the reusability of the Quality sections can vary from one region to another.
- Module 3: (Quality). While some documents may be reused, many are written for specific regions. Ask yourself…what are the specific country requirements on quality? When submitting to a new region, will newer manufacturing information be available? How does this affect Module 2?
- Module 4 (Preclinical): Most of this module can be reused, but there are specifics around how the pre-clinical study reports are added into eCTD software. In the US and Canada, Study Tagging files are created to organize the reports. In Europe, the Node Extensions are used to organize the reports. Also, there are specific dataset requirements for reports submitted to the US in submodules 4.2.3.1, 4.2.3.2, and 4.2.3.4.
- Module 5 (Clinical): A lot of this module can be reused, but there are also some key specifics that you need to be aware of. The US and Canada require Case Report Forms (CRFs), but Europe does not. The US organizes the CSR (Clinical Study Reports) components, the CRFs, and datasets using Study Tagging Files. Canada also allows for Study Tagging files but CRFs are moved to a section called 5.3.7 (Case Report Forms). Europe organizes reports by Node Extensions. Case Report Forms and datasets are not typically submitted. However, Europe can request Case Reports Forms. If they do, you only have 48 hours to turn them around. Europe also uses Module 5.3.7 to place the CRFs.
Pitfalls to avoid
- Putting application and/or sequence numbers in the headers of your documents reduces their re-usability and can require extra time to update if they are reused.
- Overlinking documents that will be removed or found in a different eCTD section for a different region can mean extra time spent on removing or resetting links.
How can I use eCTD software to make my life easier?
Don’t feel too overwhelmed. Most eCTD software applications have a “Clone” feature that allows you to convert an application from one region to another. Usually, the software will understand the regional differences and prompt you to check the conversion of study tagging files to node extensions (and vice-versa), ensure proper location of CRFs and datasets, etc. If there are extra files, you can always remove them.
Using validation software can also let you know where links may be broken so you can either remove them or reset them.
When it comes to managing your eCTD submissions, you should work with RegOps experts. At Certara, we can help you simplify your regulatory processes and assure submission success with our GlobalSubmit eCTD software and Certara Regulatory Operations and eCTD Publishing specialists.




