Getting the dose right in gene therapy (GT) is very challenging. Dose escalation within an individual is typically not feasible, and each patient can only receive a single dose since a profound immunogenic response will render subsequent administrations ineffective. Too low a dose might exclude that patient from any future benefit, while too high a dose can be very dangerous, since any side effects may be irreversible and could last for years. The definition of a narrow therapeutic index takes on a whole new meaning in gene therapy. You really need to get the dose right the first time in every patient.
There are no established pharmacokinetic/pharmacodynamic (PK/PD) modeling approaches available for gene therapy, the translational utility of pre-clinical models in the context of dose prediction is unclear, and conventional dose finding approaches from early clinical development do not apply. A combined phase 1/2 study may only consist of a dozen or so patients. Therefore, predicting inter-individual variability and the role of covariates cannot be addressed by statistics. These challenges are outlined in the regulatory guidelines for gene therapy.
Motivated by these challenges and opportunity for benefits of GT, we have developed a Quantitative Systems Pharmacology (QSP) platform using our Virtual Twin™ technology. QSP builds confidence in therapeutic modality and biological targets in context of the specific disease. It integrates principles and best practices from PK/PD with systems biology, and links pharmacological activity to modification of disease.
Case Study The building block or our platform is the igem model, which consists of six interlinked modules. Following administration of the viral vector, viral capsids dock membrane receptors and are internalized via endosomes. If they escape degradation, they reach the nucleus where a viral single strand of DNA is converted to persistent circularised episomes, which drive continuous synthesis of the target protein. We significantly modified and extended this base model, embedded it into a physiologically-based pharmacokinetic (PBPK) framework to model distribution and elimination of both the gene delivery modality itself, as well as the target protein to various organs.
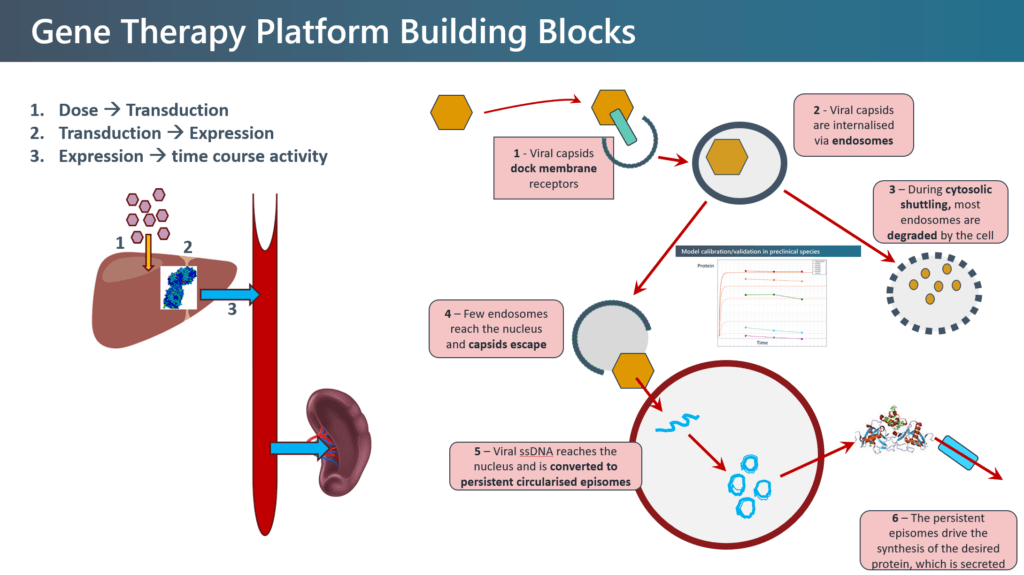
The model was calibrated with pre-clinical in vivo data and scaled to human dose predictions using Simcyp PBPK, prior to obtaining any human clinical data. When the clinical data for the first patient emerged, the model was not only refined and updated, but a virtual twin (VT) for each patient was developed. The VT is an in silico QSP clone of an actual patient using specific key characteristics, such as body morphometry (weight, body surface area, etc). A VT was created for each patient in advance of them receiving the treatment, thereby individualizing the dose. Real-time, precision medicine applied to early clinical development.
Following completion of the Phase 1/2 trial with a dozen patients, we scaled the VT approach to generate more virtual patients for phase 3 trial planning. This technology allows for the simulation of thousands of virtual patients and the simulation of virtual trials to evaluate different doses, dosing regimens and designs. We can develop VTs for different special populations, such as the elderly and pediatrics, applying the same principles of QSP-driven precision dosing.
Dose Optimization using the Gene Therapy Virtual Twin™ Platform is a novel and proven approach for real-time, iterative and personalized dosing.



