In June 2022, the FDA published their first oligonucleotide clinical pharmacology specific guidance [1]. This is a welcome addition and clarifies the position of the agency on some key aspects regarding development of oligonucleotide therapies. Whilst the guidance still leaves a few open questions, it provides drug developers with a starting point and acknowledges that oligonucleotides present some unique aspects and challenges.
In this blog, we’ll discuss the new guidance and highlight our 3 key takeaways and share our wish list for the next guidance!
But first – what is an oligonucleotide?
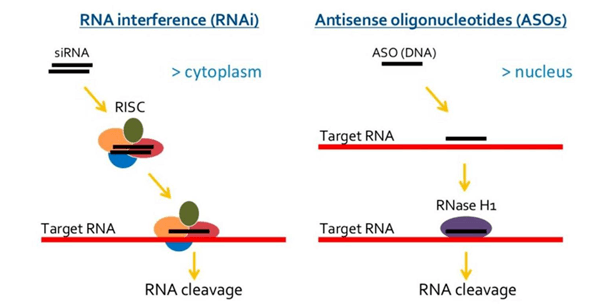
This class of single- or double-stranded small synthetic nucleic acid polymers (≈20-mer) can be used to modulate gene expression. Oligonucleotides, or “oligos” for short, modulate gene expression via various mechanisms. They can target pre-mRNA, mRNA, or non-coding RNA to induce degradation, modulate splicing events, or interfere with protein translation (Figure 1).
Currently, 15 oligos are approved across various territories for use in patients with rare diseases, with the first FDA oligo approved— fomivirsen— in 1998. Oligos have some specific pharmacokinetic (PK) properties compared to small molecule drugs. They have very low oral bioavailability (hence intravenous or subcutaneous are preferred routes of administration), high distribution in tissues (typically in the liver, kidney, spleen, and lymph nodes), no or very low blood brain penetration, rapid plasma clearance, and degradation via nucleases within tissues, which results in a very long residence time. Learn more about the PK of oligonucleotides in this blog [2].
Our top 3 takeaways from this guidance
1) Drug-drug interaction (DDI) expectations
How concerned we need to be about the DDI potential of oligos has previously been uncertain. In the new guidance, the FDA doesn’t require assessment of the oligo as an in vitro substrate for CYP450 enzymes or transporters – unless there is substantial renal active secretion, in which case they suggest in vitro assessment of renal transporters may be important. They want sponsors to assess the ability of oligos to modulate CYP450 enzymes and transporters though, and these stay recommended, even if a well-founded justification could be tested during interaction with agencies!
2) Immunogenicity assessment is expected
Immunogenicity is the ability of a foreign substance, such as a drug treatment, to provoke an immune response. Historically, the immunogenicity assessment of oligos has been somewhat patchy – with some programs including it and others not. For example, inclisiran approved in 2021 [3] included anti-drug antibody (ADA) assessment on their drug label, whereas casimersen approved in the same year didn’t [4]. The FDA guidance has clarified that they expect an immunogenicity risk assessment completed for oligos. The assessment includes a multi-tiered immunogenicity assay approach on the product itself, the carrier component, or its targeted modified protein. Whilst this will be more burdensome for sponsors who hadn’t planned for this, it makes sense from a patient perspective while we explore further this aspect of oligos.
3) No surprises regarding QTc or organ impairment
Clinical pharmacology assessments for QTc and organ impairment refer to the risk of a drug causing QT prolongation (a risk factor for cardiotoxicity; Figure 2) and the potential need for dose adjustment in patients with renal or hepatic impairment, respectively. QTc and organ impairment sections are included in the guidance. However, the recommendations are less oligo specific, and the existing guidances on QTc and renal or hepatic impairment relevant to small molecules are referred to [5-9].
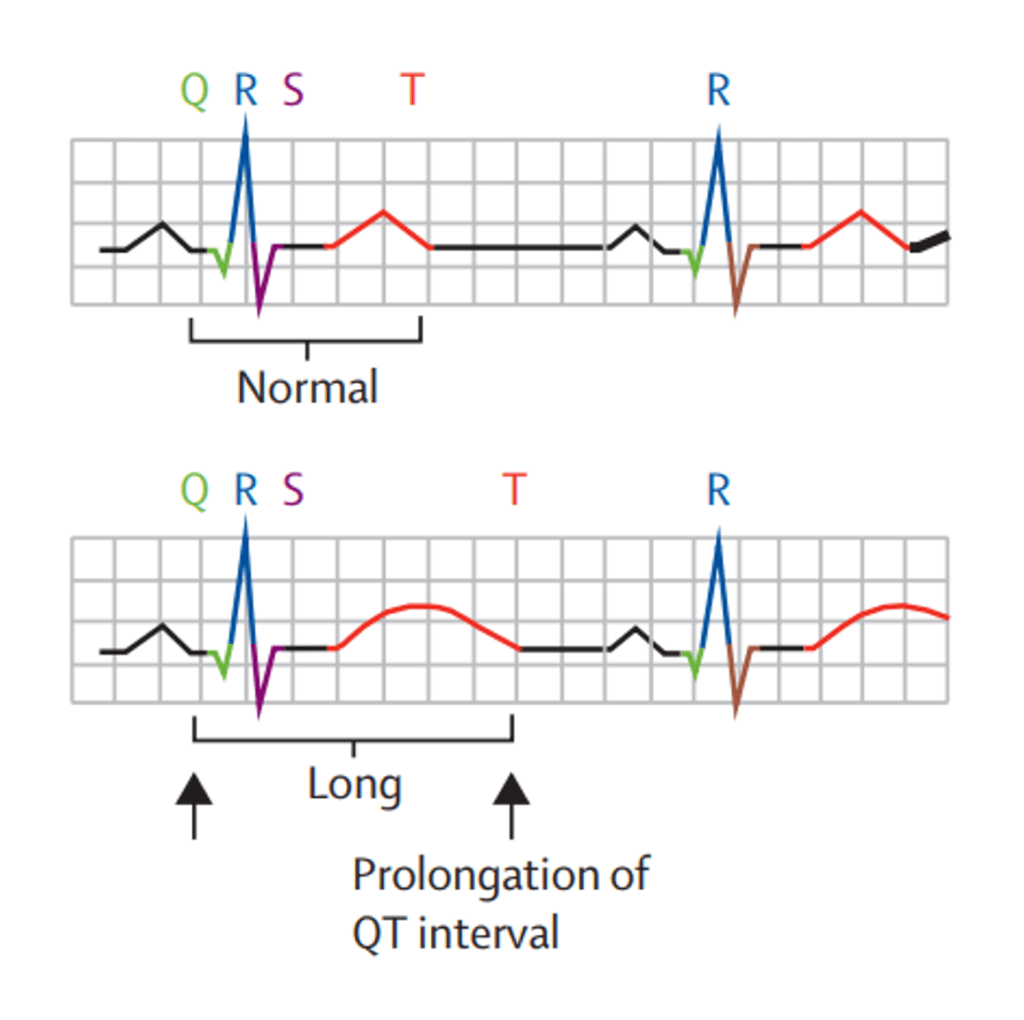
Perhaps most interesting for oligos is the potential impact of renal impairment, which is discussed in more depth in this blog [10]. Drug developers will likely continue to discuss this topic with the FDA as we gain more insight on potential impacts or otherwise.
Future thinking
This guidance has whet out appetite for more! Other areas where it would be great to firm up thinking are:
- Excretion balance in human: in the new guidance, the FDA mention identifying “the role of the liver and kidney in the disposition and elimination of the oligonucleotide therapeutic by considering in vitro, preclinical, and early Phase 1 clinical data.” Indeed, excretion balance studies are very complex whether in preclinical species or in the clinic due to very long elimination of product from the body. It remains difficult to document elimination pathways of oligos using a standard excretion balance study. Metabolism is typically via nucleases to produce chain-shortened metabolites that are not easy to quantify without a standard excretion balance study
- Bioanalysis: achieving a limit of quantification that allows measurable concentrations long enough to observe different distribution and elimination phases is challenging for this type of product.
- Use of allometric scaling: an advantage of oligos is that we can observe good interspecies allometry that proves very useful for first-in-human (FIH) dose determination. Oligos have high distribution into tissues, which can present challenges of how to relate a patient’s plasma concentrations to what’s happening at the site of action, especially when we don’t have an easy biomarker to measure and follow. Use of preclinical tissue:plasma concentration ratios can help inform on what we might expect in the clinic. This high distribution into tissues with the associated long residence time also has potential impact on safety evaluation – another area that would be worthy of further thinking
In summary – the new oligos guidance didn’t deliver many big surprises. However, it’s a big step forward to gain oligo specific guidance that provides clarity about what we need to do and don’t need to do when developing oligos. We look forward to seeing the guidance evolve over time to cover even more of the common questions we have as developers of oligo therapeutics.
Need guidance with your oligo program? Click here to learn how we’ve supported the success of 23 oligonucleotides programs, and how we can help you!




