One less-publicized provision of the Inflation Reduction Act (IRA) enacts a five-year boost in Medicare Part B reimbursement for biosimilar drugs (also known as follow-on biologics or subsequent entry biologics). The biosimilar reimbursement enhancement is meant to overcome financial disincentives to adoption.
Complete the form to download a free copy of the IRA Biosimilars Outlook 2024 Report
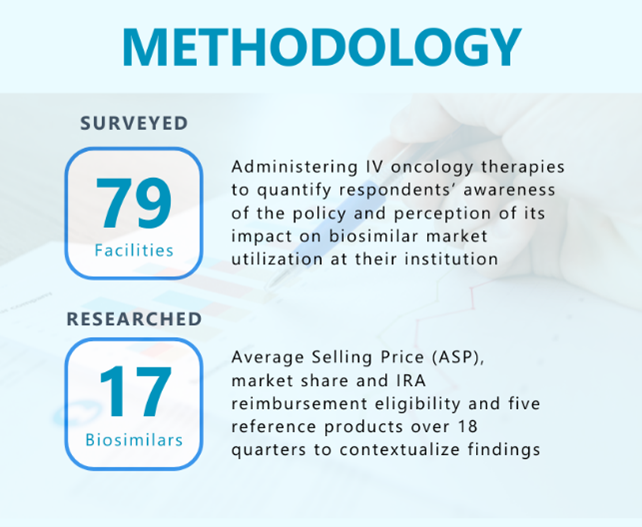
The research delves into the IRA’s “small but measurable” impact and offers insights in several key areas, such as:
- The relationship between awareness of IRA enhanced Medicare part B reimbursement and utilization of biosimilars at oncology treatment sites
- Facility characteristics associated with level of biosimilar utilization
- Relationship between eligibility for the IRA biosimilar reimbursement boost and uptake
- Drivers of and impediments to biosimilar uptake
- Trends in oncology biosimilar uptake and market share
If you’re interested in discussing this topic or other U.S. access trends with our experts, you can contact them directly.
Authors
Caitlin Verrilli, MBA, Director, US Access Strategy
[email protected]
Max Vargas, PhD, MBA, Vice President, US Access Strategy
[email protected]
Matias Junghahn, Senior Analyst
[email protected]


