Tag
pre-update
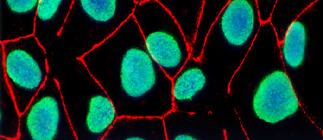
Interlaboratory Variability in the Madin–Darby Canine Kidney Cell Proteome

Madin–Darby canine kidney (MDCK) cells are widely used to study epithelial cell functionality. Their low…
CertaraJune 28, 2023
Applying Technology to Expedite Regulatory Writing

Many medical writing capabilities and approaches have matured over the past several years opening the…
CertaraJune 25, 2023
GlobalSubmit Review and You: How to Expedite the Submission Review Process

Have you ever wondered how you could speed up the submission review process without compromising…
CertaraJune 25, 2023
DIDB® – The Drug Interaction Database, is now part of Certara

PRINCETON, N.J.— June 20, 2023 — Certara, Inc. (Nasdaq: CERT), today announced it has acquired…
CertaraJune 21, 2023
Certara Appoints new Chief Marketing Officer, Sheila Rocchio

Princeton, N.J., June 13, 2023 – Certara, Inc. (Nasdaq: CERT), a global leader in biosimulation, today…
CertaraJune 14, 2023
What’s New for SEND 2023

This webinar will focus on several changes to the CDISC SEND standard that became active…
CertaraJune 12, 2023
Certara Launches Version 8.4 of Phoenix™ Biosimulation Software for Drug Development

Latest updates demonstrate commitment to customers and market leadership of Phoenix Platform PRINCETON, N.J.— June…
CertaraJune 12, 2023
Certara enhances CODEX Clinical Outcome Analytics Platform with AI

Trial design and execution now accelerated by deep learning and data fabric capabilities. PRINCETON, NJ…
CertaraJune 8, 2023
Unlocking Efficiency and Quality: What is New in Phoenix™ 8.4?

Join us for an exclusive webinar as Certara introduces the highly anticipated version 8.4 of…
CertaraJune 5, 2023