


Using MBMA to run virtual “head-to-head trials” Blog Post
Osteoporosis is common in post-menopausal women. The long-term sequelae of osteoporosis include bone fractures, particularly of the hip and vertebrae. Bone mineral density (BMD) of the lumbar spine (LS) and...
Phoenix Hosted Page
Phoenix Hosted delivers faster compute, outsourced validation, and flexibility of access across Mac and PC operating systems.
Antibody-drug Conjugates in the Clinic: Key Considerations & the Role of Modeling in Expediting Development Blog Post
Antibody-Drug Conjugates (ADCs) can be an ideal drug treatment for cancer because they deliver a cytotoxic anti-cancer drug directly to the tumor with reduced off-target damage. ADCs combine the targeting…
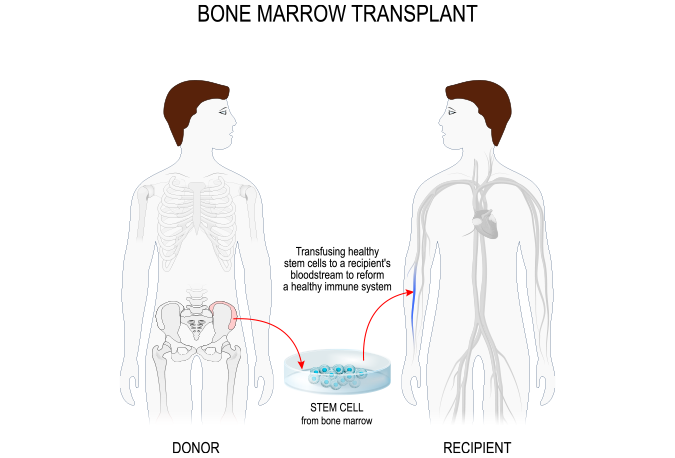
Using Real-World Evidence to Derive an External Control Arm for a Rare Disease Drug Trial Blog Post
Our client was developing a new anti-viral drug for a rare, life-threatening viral infection that affects Hematopoetic Stem Cell Transplantation (HSCT) patients. This rare disease had no approved therapies. Solutions...
Stepwise PIPs: A Solution for the Challenges of Pediatric Drug Development Blog Post
Early paediatric investigation plan (PIP) submissions – an unnecessarily bitter medicine? In the European Union (EU) Paediatric Regulation, the first PIP submission is requested early in the clinical development program….

Repurposing Data & Advanced Analytics to Validate Surrogate Endpoints Blog Post
Repurposed data and advanced analytics can be used to validate a surrogate endpoint. A surrogate endpoint is a substitute endpoint for a clinically meaningful endpoint in clinical trials. It is...
LABO: Data utilisation and data infrastructure in drug development: Data, Data, Data Blog Post
LABO: Über Datennutzung und Dateninfrastruktur in der Wirkstoffentwicklung - Daten, Daten, Daten Published: April 12, 2023 This is the German article written by Dr. Fabian Rauscher, Scientific Informatics Manager, Europe,...



