
Understanding the Impact of FDA Modernization Act 2.0 on Drug Development Blog Post
Learn how the FDA Modernization Act 2.0 will change the drug development landscape by allowing alternative methods to animal testing.

Learn how the FDA Modernization Act 2.0 will change the drug development landscape by allowing alternative methods to animal testing.

Wednesday, August 9th 2023 More than 50 bispecific antibodies are in oncology clinical development with a large diversity in formats, directed at a range of immune and tumor targets. Bispecifics…
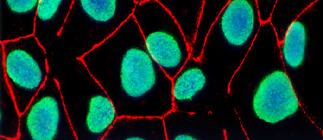
Madin–Darby canine kidney (MDCK) cells are widely used to study epithelial cell functionality. Their low endogenous drug transporter protein levels make them an amenable system to investigate transepithelial permeation and…
PRINCETON, N.J.— June 20, 2023 — Certara, Inc. (Nasdaq: CERT), today announced it has acquired Drug Interaction Solutions from the University of Washington. This transaction brings a world-renowned team of…


Princeton, N.J., June 13, 2023 – Certara, Inc. (Nasdaq: CERT), a global leader in biosimulation, today announced the appointment of Shelia Rocchio to the position of Chief Marketing Officer. In this…

This webinar will focus on several changes to the CDISC SEND standard that became active in March of 2023 and what these changes mean for dataset creators and users. Changes…

Latest updates demonstrate commitment to customers and market leadership of Phoenix Platform PRINCETON, N.J.— June 12, 2023. Certara®, the global leader in biosimulation, today announced the launch of version 8.4…

Trial design and execution now accelerated by deep learning and data fabric capabilities. PRINCETON, NJ June 8 — Certara, Inc. (Nasdaq: CERT), a global leader in biosimulation, is integrating new…

Certara’s support, from dose optimization to medical writing, helps pharmaceutical companies advance their research, make informed decisions & publish their research.