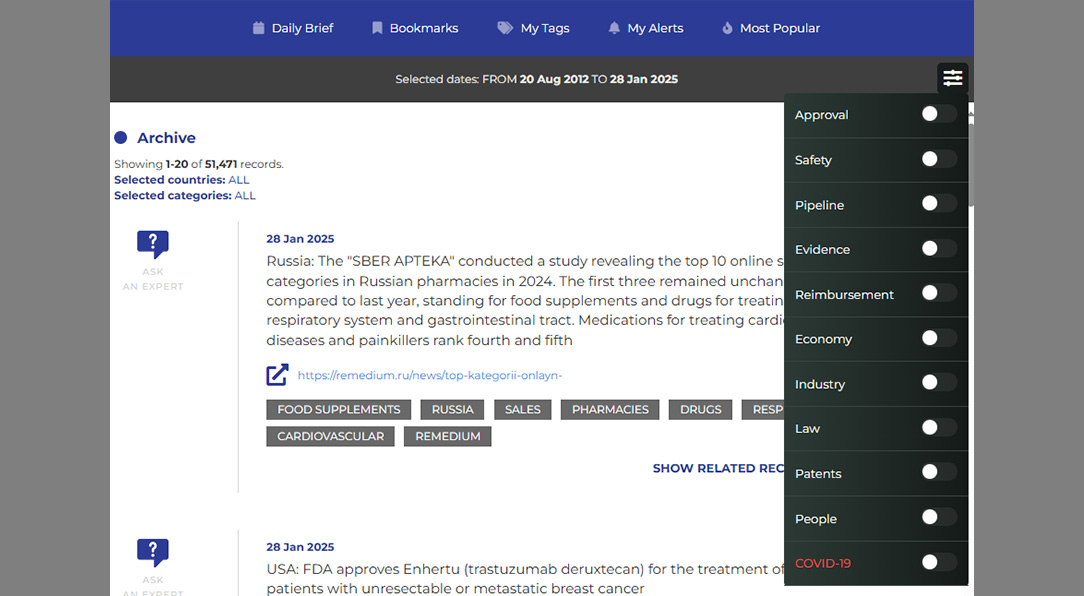
Certara’s Market Access Radar (MAR) is the industry’s leading market access repository, designed to streamline access to critical pricing and reimbursement data. MAR consolidates the latest market access news and insights into a single, interoperable platform, enabling organizations to make informed decisions with confidence. With data sourced from over 50 countries and updated daily, MAR ensures you stay ahead in the dynamic landscape of market access.
Market Access Radar
Daily Delivery of Global Market Access News and Reports
Learn more about Market Access Radar
600
50
Quick and easy access to the most important information
Market Access Radar (MAR) is your complete market access repository, offering a secure and consistent workflow to manage and analyze pricing and reimbursement data. Its intuitive interface allows users to visualize data flow and customize workflows for efficient decision-making. MAR is designed to save time, enabling you to focus on strategy rather than searching for information.
Daily updates on critical market access news from over 600 sources.
Insights into payer and HTA decision-maker actions and policy changes.
Automated workflows that can be saved, customized, and shared.
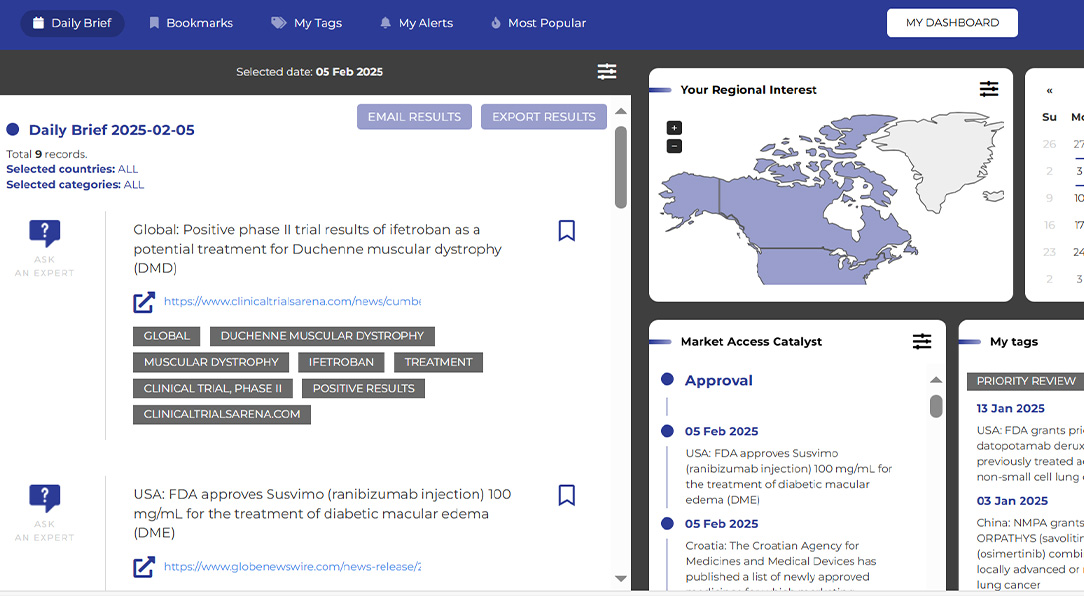
Create and experience according to your requirements and interests
A look at the market access repository’s key capabilities
Daily brief
Customized email news update based on criteria selected in the MAR platform
Tailored exports
Quick and easy export of search results and profiles
Ask an expert
Send a question on any MAR record directly to a Certara expert
Profile creation
Set up a personalized profile based on your interests and requirements
Manage records and tags
Select tags and alerts to customize your feed and dashboard
HTA Updates
Latest reimbursement recommendations and news from HTA agencies
Market access news
A mix of regulatory and reimbursement news from EMA, HMA, FDA and MHRA
Reimbursement Data
Recommendations and information on financing from public funds
Why Certara
Certara empowers organizations with innovative tools and expert insights to navigate the complexities of market access with confidence.
Trusted by leading pharmaceutical and biotech companies worldwide.
Over 20 years of experience in market access and regulatory solutions.
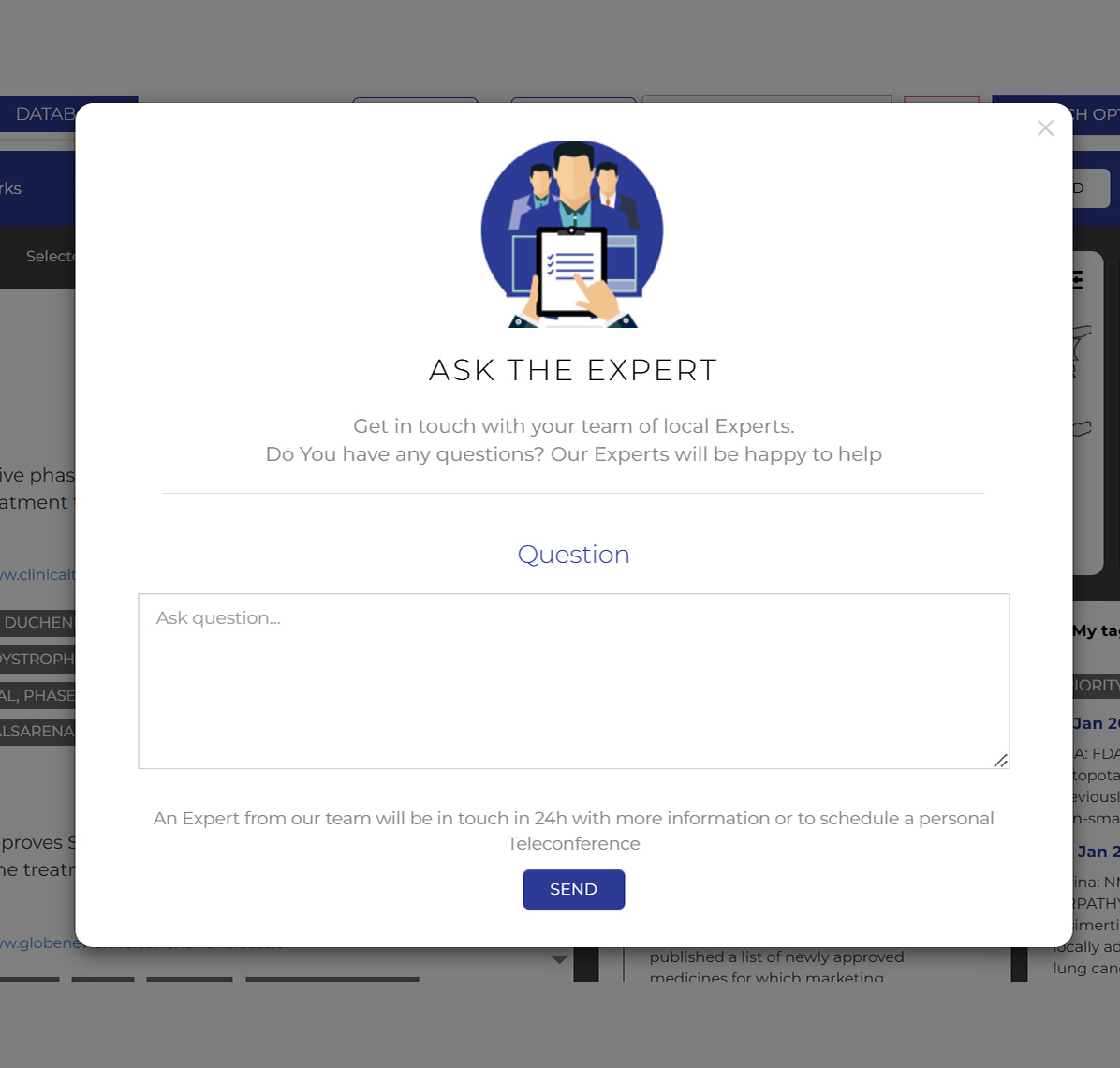
Expert analysis for any topic
Submit a question about any record found on the Market Access Radar directly to a Certara Market Access expert.

Schedule a Demo
Experience the power of Certara’s Market Access Radar firsthand. Request a demo today to see how the market access repository can transform your market access strategy.
Gain access to the latest market access news and data.
Streamline pricing and reimbursement data management.
Leverage expert-curated insights for strategic decision-making.
FAQs
What is Market Access Radar (MAR)?
MAR is a comprehensive Market Access Repository that consolidates pricing and reimbursement data from over 50 countries.
How often is the data updated?
Data is updated daily, ensuring you have access to the latest market access news and insights.
Can workflows be customized?
Yes, MAR allows users to create, save, and reuse customized workflows for efficient data management.