One of the most common questions posed by clinical operations experts when including pharmacokinetic sampling in a clinical trial is the following:
“What is the time window we should allow for each blood sample?”
My answer is always the same: “Don’t include any window.” I am almost always met with a confused look. The confusion is borne out of years of social programming for clinical operations staff by inexperienced clinical pharmacologists. I still see wording in protocols that say something similar to, “Because PK is the primary endpoint, the blood draws for PK samples should take priority over all other activities and should occur on the scheduled time point before any other procedure.” Unfortunately, statements and sentiments such as these are not supported by scientific data and actually can contribute to poor quality clinical data. Using a simple example, I will demonstrate that sampling time does not affect PK parameter calculations.
Pharmacokinetic analysis requires data pairs that consist of a sampling time and a drug concentration. These data pairs are then used in a PK analysis (normally non-compartmental analysis) to calculate PK parameters and drug exposure. A proper PK analysis requires a complete data pair (time, concentration). The interesting part is that one portion of the pair (concentration) is dependent upon the other (time). Thus, a change in time will automatically cause a change in concentration. For example, if two blood samples are drawn at 1.0 (t1) and 1.25 (t2) hours after dose administration, the concentrations associated with those times exist such that C1 > C2 during the elimination phase, and C1 < C2 during the absorption phase. And the concentrations of each can be predicted based on the function C(t).
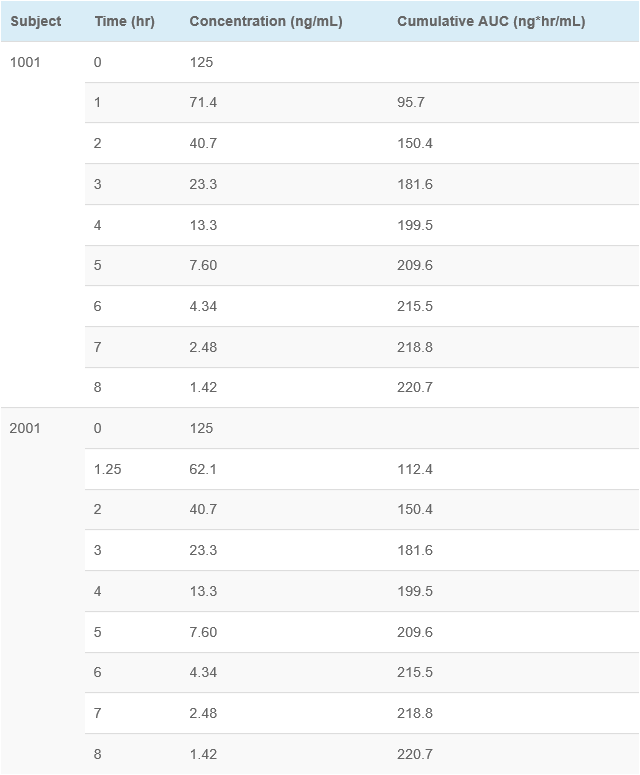
Effect of Sampling Times on AUC Calculations
This example shows concentration-time data for two subjects. Subject 1001 had blood samples drawn at the scheduled (nominal) time points. Subject 2001 had the 1 hour sample drawn at 1.25 hours (15 minutes late, which would normally be a protocol deviation using “windows”), and then all other samples taken at the scheduled time points. As you can see, the cumulative AUC was identical (220.7 ng*hr/mL) for both subjects. This supports the conclusion that exact blood draw times are not needed for accurate assessment of PK parameters.
This means that setting sampling “windows” does not affect the PK parameters and only leads to extra work for the clinical and data management teams as they verify the time of the blood sample versus the acceptable window, record any deviations, and then supply justification for the deviations. All of that extra work for absolutely no advantage. The PK results are the same whether the samples are “in window” or not. Additionally, when PK blood sampling is given priority, it can negatively affect other assessments such as vital signs, ECG measurements, and behavioral measures. And lastly, with such an intense focus on getting a sample at a specific time, often clinical staff record incorrect blood draw times to stay “in window” rather than report the actual blood draw times. These incorrect blood draw times will have a negative impact on the PK analysis as their are errors in the data pairs (incorrect time associated with measured concentration).
So how do I write my protocols? I list nominal sample times, and then I add language similar to this: “Clinical staff is encouraged to take the blood samples for PK analysis at the scheduled time point; however, deviations from the scheduled sample times are not considered protocol deviations. The exact time and date of the blood draw must be recorded using an unambiguous format such as DD MON YYYY and HH:MM using a 24-hour clock.” Using this language, the clinical staff is free from worrying about sampling windows, and focuses on recording exact times. I also provide a priority for procedures scheduled at the same nominal time point (eg, vitals, ECG, blood draws).
On your next protocol, rethink your blood draw procedures and language. Perhaps you can simplify the language and avoid unnecessary work which will improve data quality, reduce costs, and make life a bit easier for everyone.
In pediatric clinical studies, only sparse blood samples can be collected. Watch this webinar to learn how modeling and simulation was used to design an adaptive trial with minimal PK sampling timepoints.