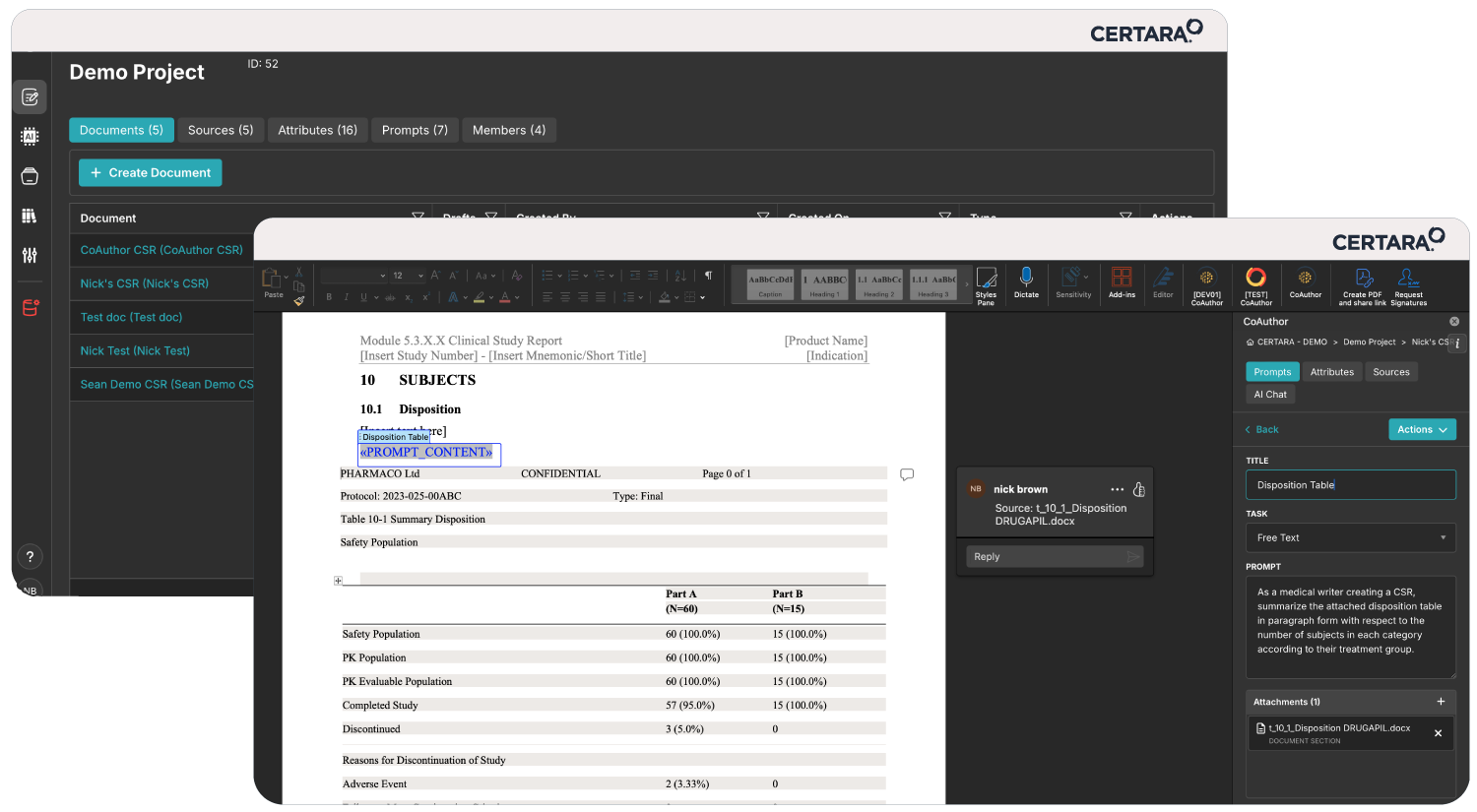
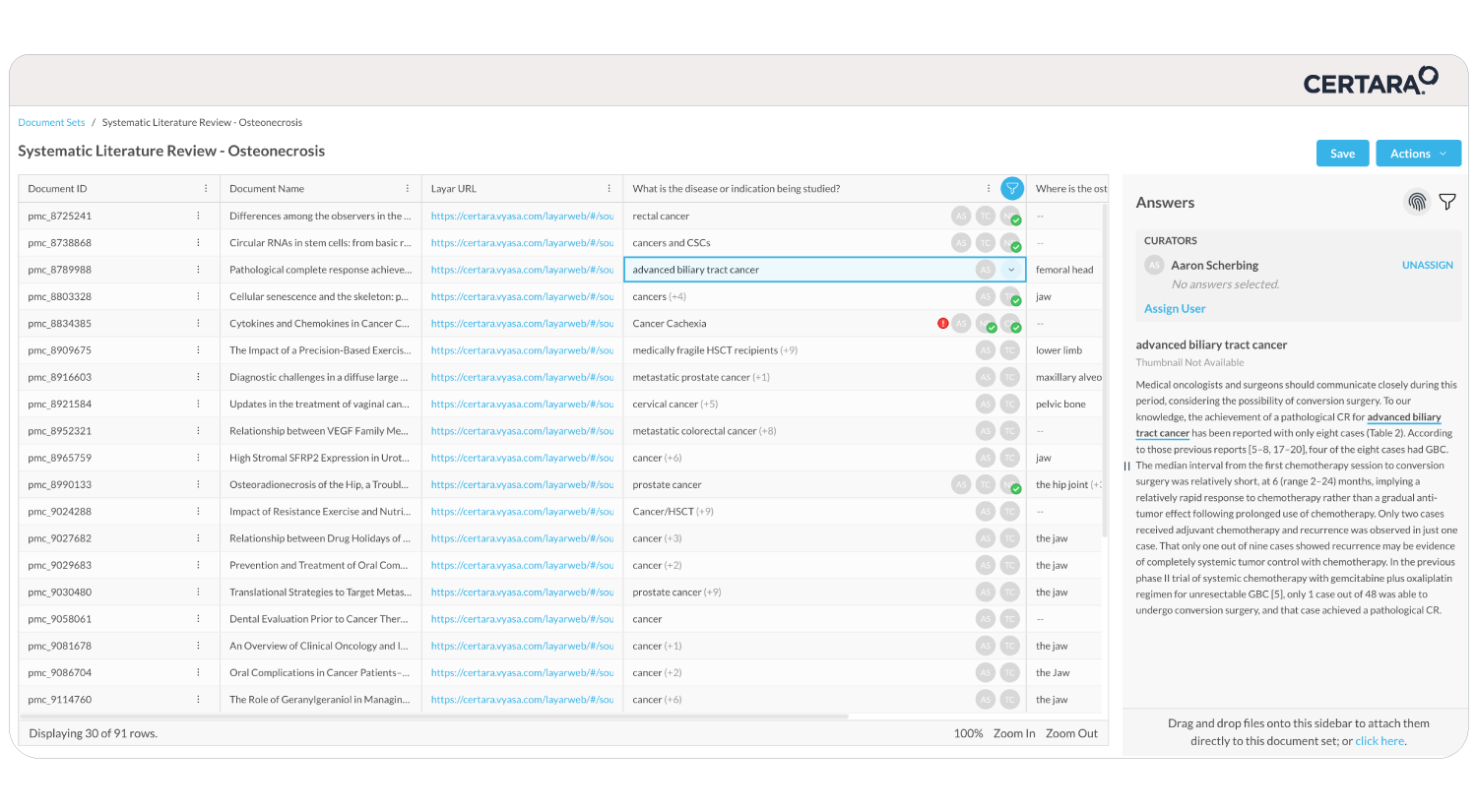
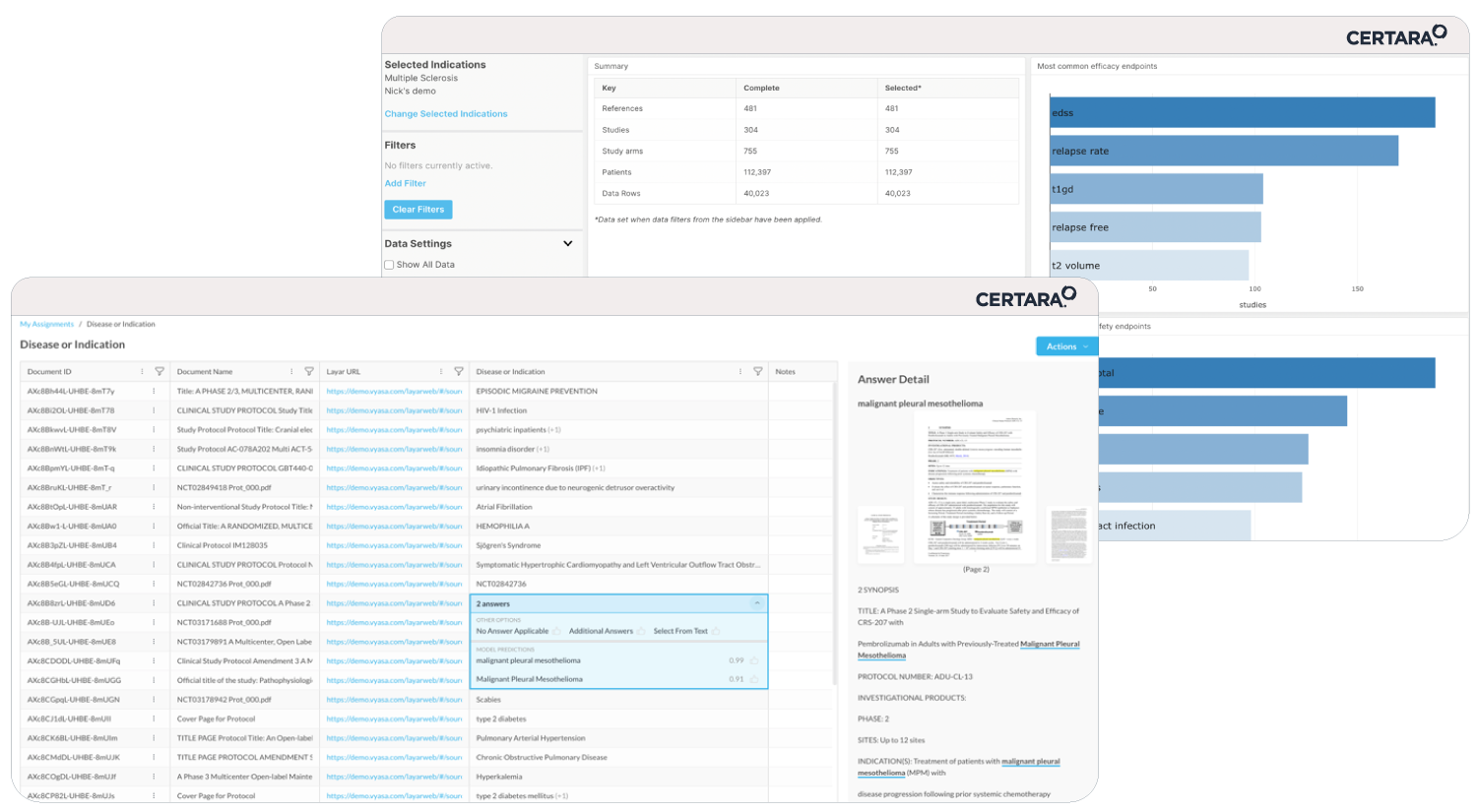
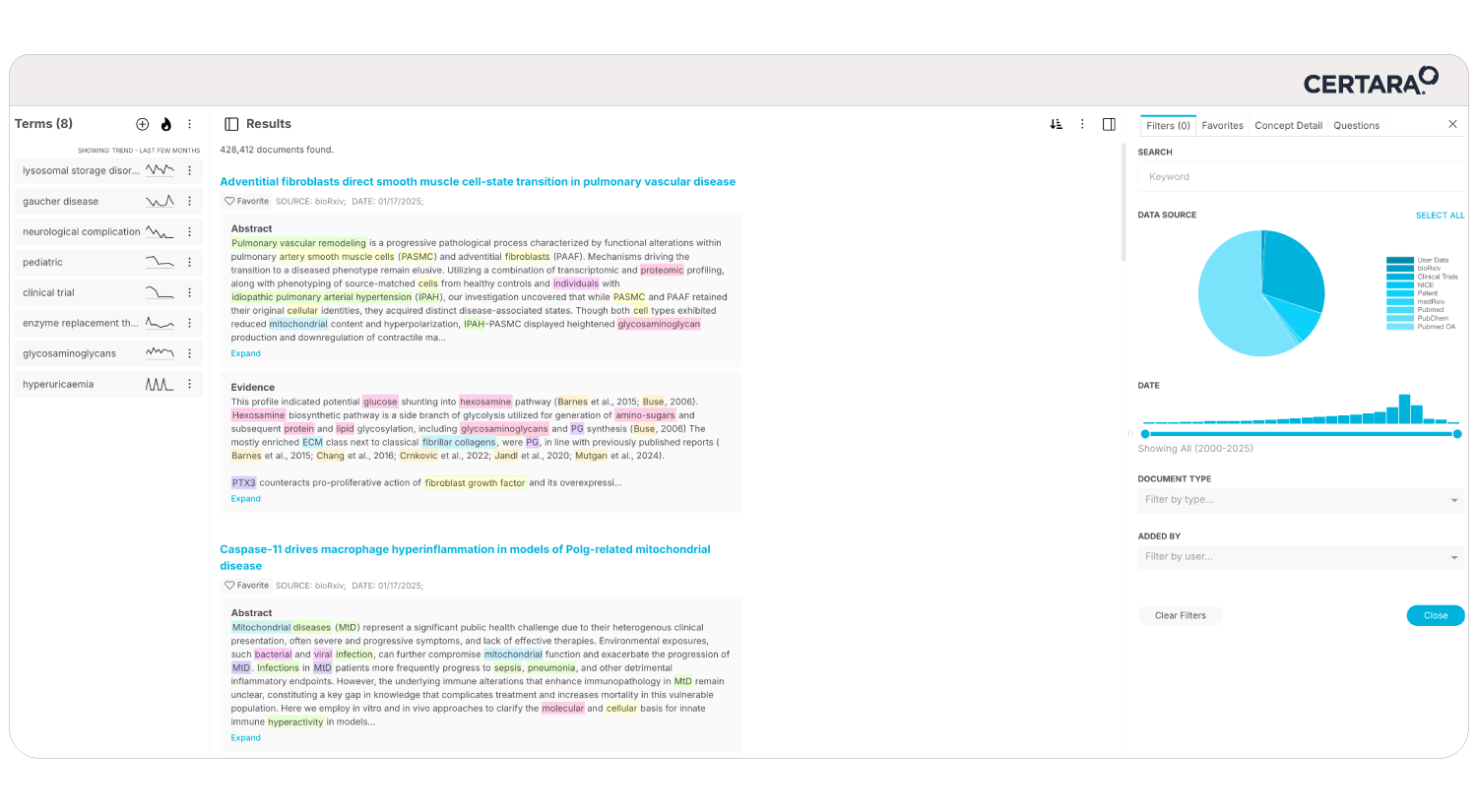
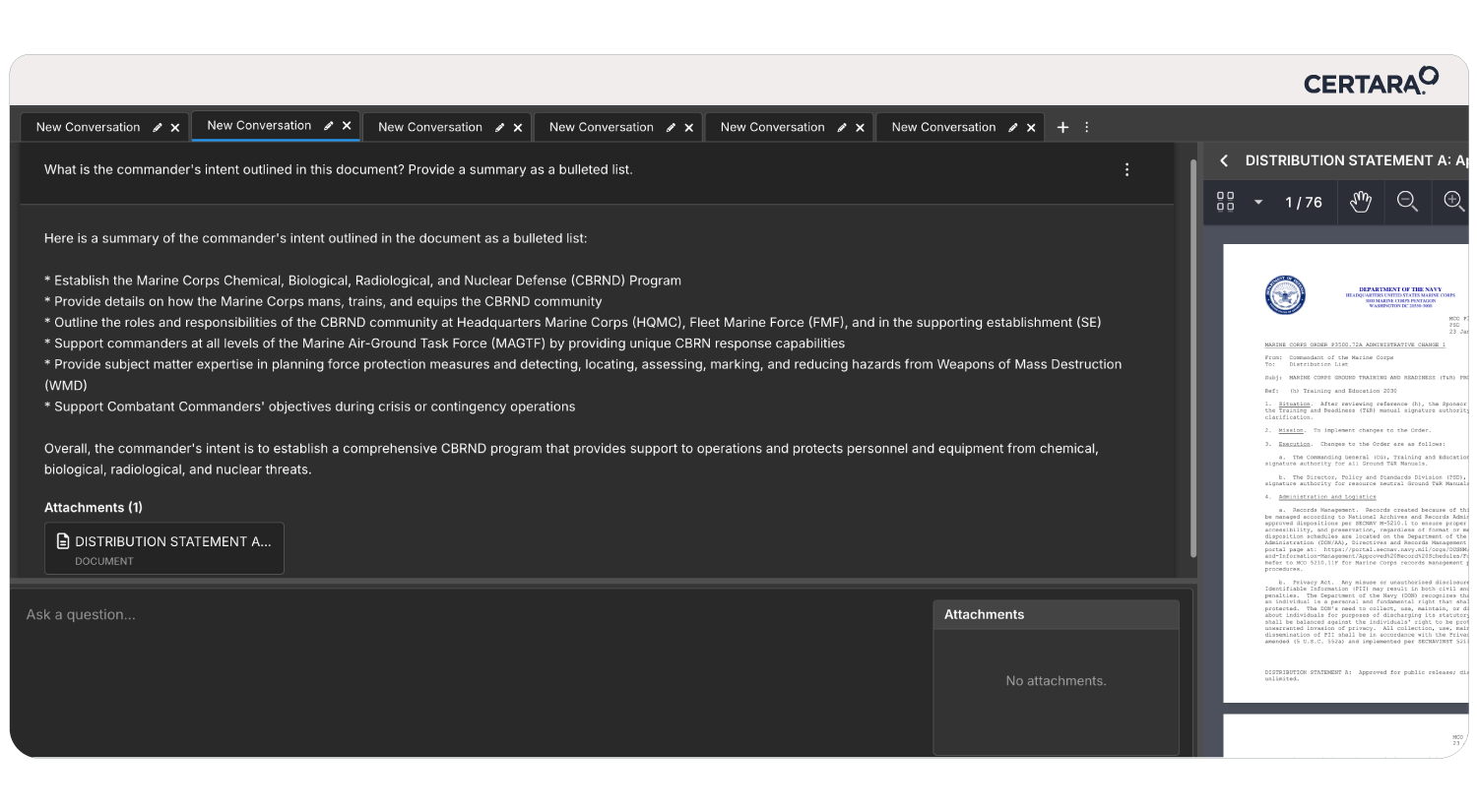
Meeting shrinking submission timelines while innovating faster on limited resources can feel overwhelming. That’s where CoAuthor, our GPT-powered regulatory writing platform, steps in. Designed to address the evolving demands of pharmaceutical companies, CoAuthor transforms the way regulatory teams draft and manage submission dossiers.
Here’s how CoAuthor accelerates your workflows:
- Faster Dossier Drafts: Streamline the creation of submission documents, including Modules 1–5, reducing timelines.
- AI-Powered Automation: Leverage secure, organization-specific GPT models to automate first-draft creation while ensuring compliance.
- Effortless Multitasking: Manage multiple program documents and source data seamlessly within a single, cohesive platform.
With CoAuthor, regulatory writing evolves from a time-consuming bottleneck into a streamlined, efficient process—empowering your team to move faster without compromising quality or security.