A US-based university with a nationally recognized academic health center implemented Clinical Data Interchange Standards Consortium (CDISC) standardization to ensure regulatory compliance and future funding success.
Challenge 1: Retrospective SDTM mapping
The need for CDISC compliance really became apparent for the university when they submitted a study for Mirdametinib, a MEK inhibitor, just before the CDISC mandate came into force. The Food and Drug Administration (FDA) noted that the collected data was not CDISC compliant.
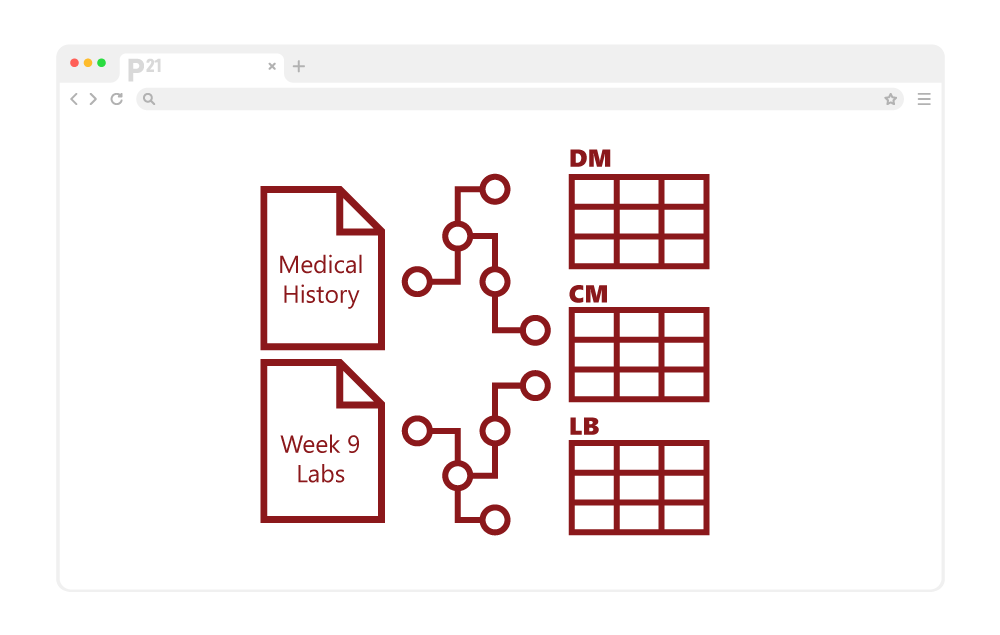
The university was faced with retrospectively mapping the study to CDISC’s Study Data Tabulation Model (SDTM), which was no easy task. Because the data had been collected without SDTM in mind, they found terminology inconsistencies and gaps in the required data.
Challenge 2: A cultural shift towards CDISC compliance
The university faced many challenges on their journey to CDISC standards implementation:
- Lack of CDISC knowledge internally – Particularly when it came to collection and submission requirements.
- The cultural shift of adopting the new methods – CDISC standardization was a big shift away from the norm for the university, which had run things differently for many years.
- Budget to invest in standardization, including budget for training – In departments with stretched budgets and busy staff, the amount of work and training required to implement standardization was a deterrent.
Learn more about Pinnacle 21
The Pinnacle 21 (P21) next generation cloud suite optimizes the end-to-end clinical trials process. Increased efficiency, maximum data quality, and improved data flow across stakeholders are now at your fingertips!
· Standardize and reuse content in your Clinical Metadata Repository
· Design and build EDC studies in under six weeks
· Get quality third-party data insights, on time
· Increase the speed and quality of your clinical trial submission data

Contact us





