Highlights
⬇️ Who are CDISC?
⬇️ Where our partnership began
⬇️ Encouraging the adoption of CDISC standards
⬇️ Visualizing CRFs for EDC Systems
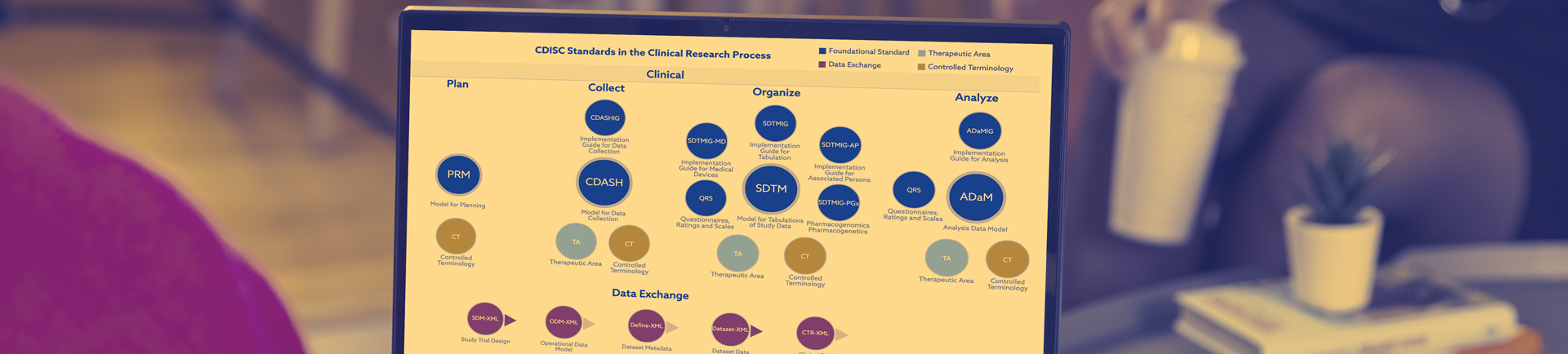
Who are CDISC?
The Clinical Data Interchange Standards Consortium (CDISC) began in 1997 as a grassroots initiative, in response to the need to better structure and improve the quality and consistency of data in clinical research. Today, CDISC is a global nonprofit charitable organization 501(3)(C), headquartered in Austin, Texas, with the CDISC Europe Foundation located in Brussels, Belgium.
What does CDISC do?
CDISC develops vendor-neutral, consensus-based, community-developed standards that enable the accessibility, interoperability, and reusability of data.
The following CDISC standards are required for submissions to the United States Food and Drug Administration (FDA) and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA):
-
- Study Data Tabulation Model (SDTM)¹
-
- Analysis Data Model (ADaM)²
-
- Standards for the Exchange of Nonclinical Data (SEND)³
-
- Define-XML⁴
CDISC standards are freely available on the CDISC website.
The consortium brings together a community of members across the clinical research lifecycle – from researchers to reviewers. CDISC collaborates with global partners, like us, so they can continue to advance interoperable data standards, achieve greater clarity, and enable more powerful research.
Proud to be one of CDISC’s key long-term partners!
In this case study, we will explore our 23-year partnership with CDISC. We will take a look at a number of collaborative projects that have driven the development and implementation of CDISC standards, to the benefit of all stakeholders in the clinical trial enterprise, including patients.
Where the partnership began
We were among the first CDISC members over 20 years ago. Today, we are a proud and active member of the CDISC Data Exchange Standards team.
In the early days, CDISC created the Operational Data Model (ODM)⁵ to outline how studies should be structured. At the same time, we were defining technologies for rapid study build.
Bringing real-world experience
In 2003, Kevin Burges, now Senior Product Manager at Certara, joined the CDISC team as a reviewer for ODM specifications. Kevin brought “real world” experience from working with customers, and added direct value to the standards development process.
In 2004, Kevin received an outstanding achievement award from CDISC in appreciation of his dedication and hard work in developing the standards with the ODM team.

Encouraging adoption of CDISC standards
By the 2010s, CDISC was focusing on increasing adoption of the standards to support faster, easier, higher quality data submissions to global regulatory authorities.
As our client base grew, Kevin became a key champion for the CDISC models. He understood why they were so crucial and how they could enable efficient, better-quality study build. He also saw how important it was to ensure semantic interoperability, and how the models could facilitate getting medicines to patients who need them faster.
In talking about the power of CDISC standards, Kevin says:
“CDISC brought together traditionally siloed functions (i.e., data managers, biostatisticians, clinicians, and terminologists) during the standards development process, which resulted in quality, implementable standards. CDISC encouraged stakeholders to think in advance about the studies they were designing, the outputs, and the best ways to achieve success.”
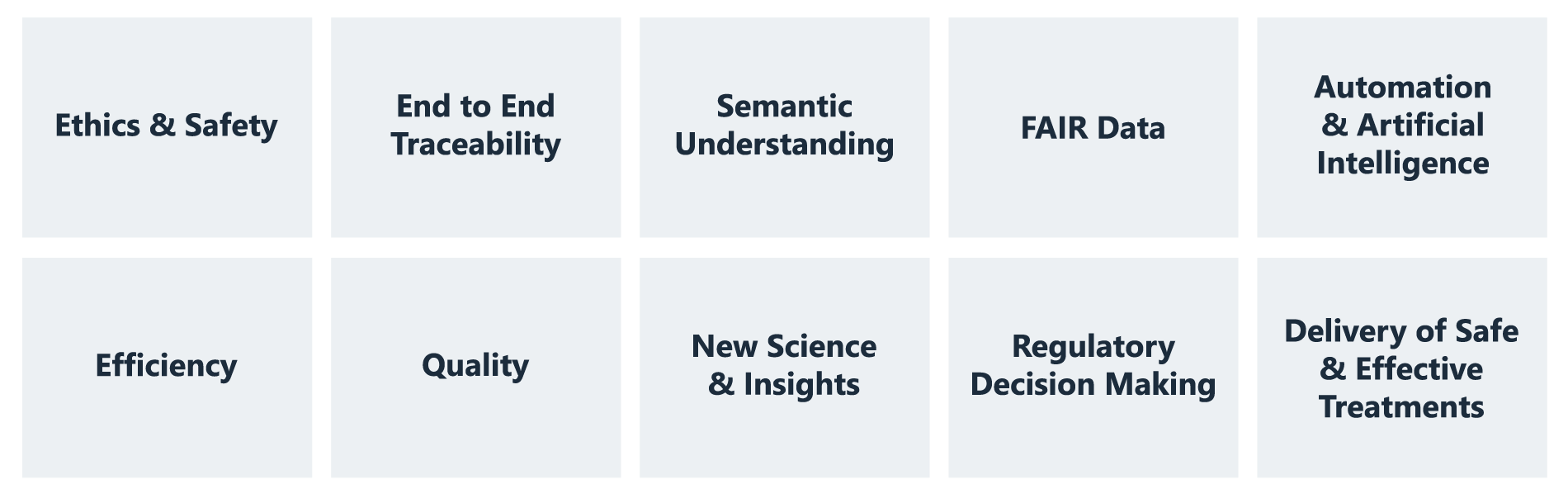
The benefits of adopting CDISC standards
CDISC standards provide a common structure and terminology for data collection, aggregation, analysis, and transfer. There are many benefits of adopting CDISC standards, including supporting the following:
Visualizing CRFs for EDC Systems
Along with the promise of creating implementation standards, CDISC faced the challenge of encouraging a risk-adverse pharmaceutical industry to shift away from traditional methods of data collection.
In the early days of CDISC standards, electronic data capture was still a relatively new concept; in our experience, around 70% of studies we were involved with still used paper case report forms (CRFs).
Easing standards adoption
CDISC knew that the key to wider standards adoption was to minimize barriers and make the standards easier to implement.
In 2011, they created a set of standardized CRFs aligned to SDTM, called CDASH. In 2021, to simplify the adoption of CDASH, CDISC decided to create an eCRF Portal, to make the CDASH content more easily accessible in human readable and machine readable forms. This would encourage use, drive better data quality, and make SDTM conversions more straightforward.
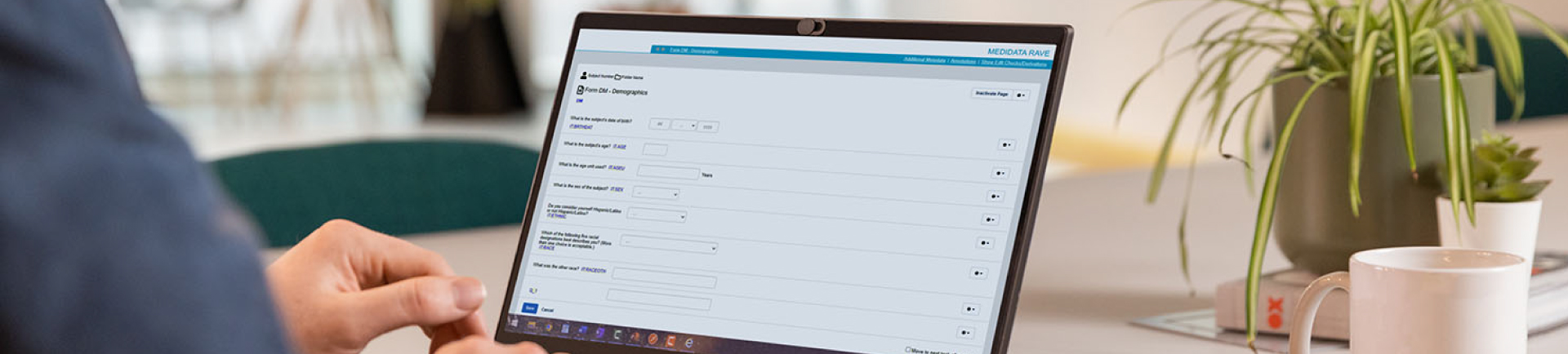
Visualizing forms before data collection
At the time, CDISC was using a metadata repository to create and store forms. However, they realized a platform was needed to visualize forms in an electronic data capture (EDC) system before patient data was collected.
By this point, we had more than two decades of experience working with CDISC standards. We had recently launched our clinical metadata repository – a centralized, cloud-based platform for creating standards and forms that generates CRF visualizations.
We were already using this technology to define, standardize and visualize eCRF metadata, so it was clear that the same mechanism could be used to create an ODM-based implementation of a set of standardized CDASH forms.

Using our platform to create, visualize, edit and approve eCRFs
CDISC used our software to create a suite of ready-to-use, CDASH-compliant, annotated eCRFs.
CDISC could easily create ODM implementations of the CDASH forms, and existing visualizations in our platform were used to provide HTML and PDF previews of what the CRFs look like and how they map to SDTM.
The platform demonstrated how the new collection of metadata could be leveraged to generate visualizations of what forms will look like in an EDC system, including SDTM annotations.
CRF forms can be exported from our platform into leading EDC systems, including:






These are freely available to download from the CDISC eCRF portal today. They’re also available directly within our clinical metadata repository. The templates can also be exported as SDTM-annotated CRFs in PDF format, as well as various HTML and XML formats.

References

CDISC standardization can help streamline your clinical metadata management. Download our comprehensive guide to CDISC standards management to learn more.


