2022 is shaping up to be the “year of the guidance” as the FDA has just released a new guidance on using circulating tumor DNA in early clinical drug development.
In this blog, I will reflect on why this agency guidance is so important for new oncology therapeutics.
Despite many important advances in oncology drug development, considerable work still needs to be done for identifying clinically meaningful circulating biomarkers that can not only serve as diagnostic aids but serve as a biomarkers of interest in understanding new drug mechanisms of action, supporting choice of indications, and optimizing dosing. With the advent of new tools, there is now an increased awareness of how genetic alterations can be a critical determinant of tumor development and progression. Many cancers are characterized by unique genetic alterations. These alterations can include single-base insertions, substitutions, deletions, and translocations with specificity to tumor cells as compared with normal cells. Two types of tumor DNA can be measured in the circulation: cell-free circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs). ctDNA is a composite of nucleic acid fragments that are not associated with cells whereas CTCs are intact cells.
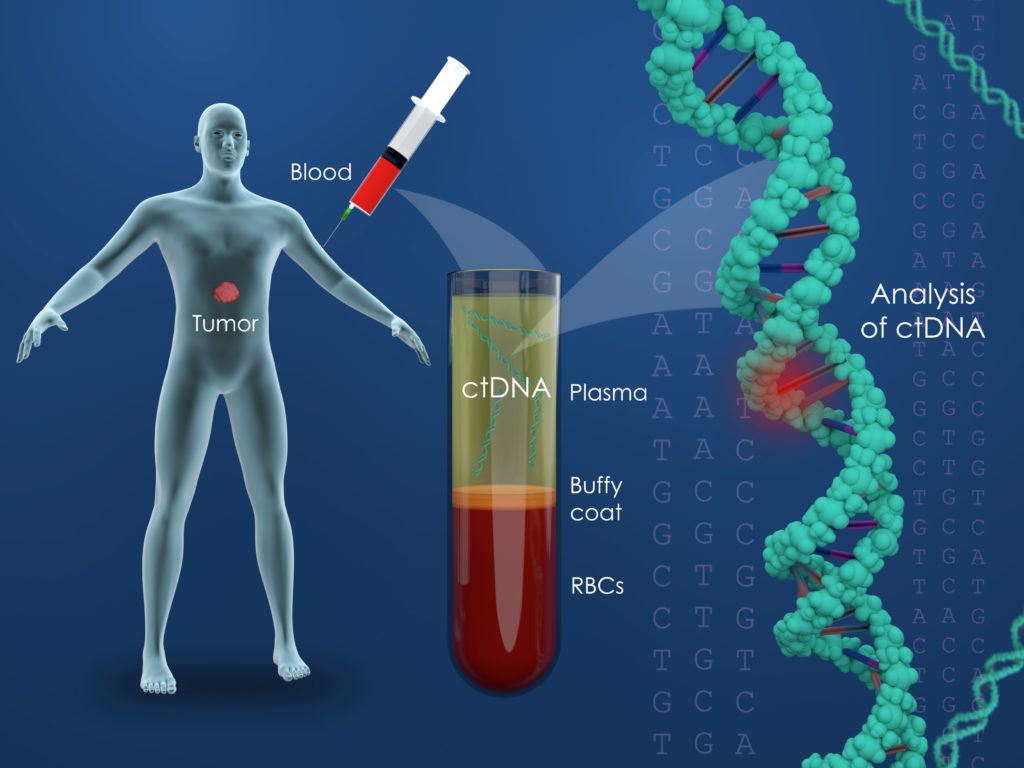
ctDNA quantification is also known as liquid biopsy. Tumors release single or double stranded ctDNA upon cellular death. Tumor DNA is thought to be shed into the systemic circulation via apoptosis or necrosis. ctDNA can be measured by various methodologies. The early assays were PCR-based and included droplet digital PCR as a gold standard while more recent methodologies employ next-generation sequencing.
The value of ctDNA measurements lie in quantifying tumor mutational burden. They could also be helpful in delineating tumor progression and in this regard, can be used as both a diagnostic tool as well as a key component of exposure/response relationships. In cases where post-dose biopsies are difficult to obtain, ctDNA can be used as a surrogate of tumor level dynamics for new therapeutics.
Why is this new FDA Circulating Tumor DNA guideline important?
For one, it elevates the use of ctDNA to measure therapeutic response. Secondly, ctDNA can be used as an early endpoint in clinical trials when its relationship with overall survival (OS) is presumed and opens an area of opportunity.
The other two cases the agency reflects upon in the guidance concern clinical trial patient selection and enrichment. The agency’s guidance also speaks to the using investigational devices it relates to ctDNA. The FDA approved the companion diagnostic test, therascreen® PIK3CA RGQ PCR Kit, (QIAGEN Manchester, Ltd.) to select patients who have PIK3CA mutations in tumor tissue specimens and/or in circulating tumor DNA (ctDNA) isolated from plasma specimens, as part of the alpelisib approval (PIQRAY, Novartis Pharmaceuticals Corporation) [FDA, 2019].
SOLAR-1 (NCT02437318) was a randomized, double-blind, placebo-controlled trial of PIQRAY plus fulvestrant versus placebo plus fulvestrant in 572 patients with HR-positive, HER2-negative, advanced or metastatic breast cancer whose disease had progressed or recurred on or after an aromatase inhibitor-based treatment (with or without CDK4/6 combination). Overall, 60% of enrolled patients had tumors with one or more PIK3CA mutations in tissue, 50% had liver/lung metastases, and 6% had previously been treated with a CDK4/6 inhibitor. 341 patients were enrolled by tumor tissue in the cohort with a PIK3CA mutation and 231 enrolled in the cohort without a PIK3CA mutation (for more details refer to the PIQRAY package insert ).
Let’s examine the relationship between ctDNA and overall survival.
Zou and coworkers (2021) evaluated the association between ctDNA metrics and the primary endpoint of OS in a subset of previously treated patients with locally advanced or metastatic non-small-cell lung cancer, treated with atezolizumab or docetaxel from a phase 3 study. ctDNA was measured by allele frequency and mutant molecules per milliliter (MMPM). Their findings showed that for patients with ctDNA median MMPM levels of < 4.79, the median survival time exceeded 17 months in docetaxel-treated patients, and the median survival time was not reached in the atezolizumab-treated patients. Their conclusion was that lower ctDNA level is associated with longer survival. There are several other studies where the association between ctDNA and OS was less compelling.
Perhaps the most proximal use of ctDNA relates to clinical disease progression in individual patients. Look at the figure 1 below for example.
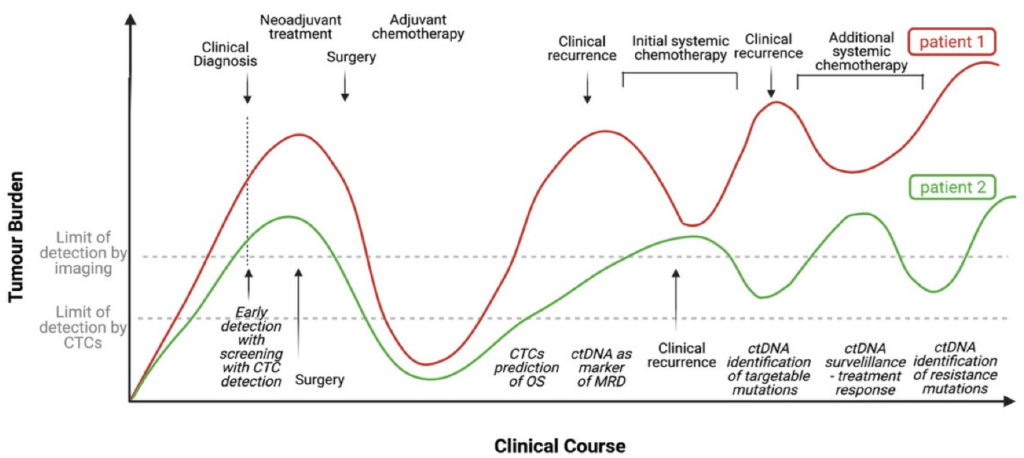
The figure shows clinical time course in two hypothetical patients. Notice that the tumor burden varies with time. It is true that ctDNA lends itself for more longitudinal analyses and potentially disease progression type analyses. It would be interesting to see the evolution of such tools to characterize exposure/response relationships. Certara scientists have state of the art experience in model-informed drug development solutions for oncology including longitudinal and disease progression modeling. To learn how we’re using these approaches to optimize oncology dosing, please visit this webpage:
Project Optimus
References
Di Capua, D.; Bracken-Clarke, D.; Ronan, K.; Baird, A.-M.; Finn, S. The Liquid Biopsy for Lung Cancer: State of the Art, Limitations and Future Developments. Cancers 2021, 13, 3923. https://doi.org/10.3390/cancers13163923.
FDA, 2019. FDA approves alpelisib for metastatic breast cancer. URL: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-alpelisib-metastatic-breast-cancer (last accessed: May 24, 2022).
Zou W et al. ctDNA Predicts Overall Survival in Patients With NSCLC Treated With PD-L1 Blockade or With Chemotherapy. DOI: 10.1200/PO.21.00057 JCO Precision Oncology no. 5 (2021) 827-838. Published online May 12, 2021.



