Health Canada’s Public Release of Clinical Information (PRCI) initiative underscores the importance of transparency in clinical research. It aims to make anonymized clinical trial information for approved drug and medical device applications accessible to the public and scientific community. As sponsors navigate this regulation, understanding non-compliance penalties is as crucial as knowing the steps for a successful submission. Here are our recommendations for planning and insights on compliance and the consequences of non-compliance.
Timelines
Health Canada’s timeline for publishing clinical submission documents is 120 days from the regulatory decision. While this may appear to be ample time, some factors can affect meeting this deadline. These may include the number of documents to anonymize, the anonymization method, and whether the study is an unexpected retrospective request. Have a plan in place as 120 days can pass quickly!
Anonymization Approach: Beyond Redaction
Health Canada strongly encourages a quantitative approach to data anonymization. Their guidance is to establish a risk threshold of 0.09 by anonymizing variables that could lead to participant re-identification. Essentially, this means that the risk of participant re-identification is approximately 1 in 11. This approach requires expertise and technology that not all sponsors have.
This shift from traditional redaction aims to protect both personal information (PI; also known as Protected Personal Data; PPD) and confidential business information (CBI). While requiring advanced expertise and technology, these anonymization approaches offer better data utility than redaction (read more about Anonymization vs. Redaction). Sponsors should adapt to these standards (C.08.009.3/C.08.012.3) to ensure compliance and data integrity under the PRCI initiative. Standard C.08.009.3 states that “the Minister may disclose, without notifying the person to whose business or affairs the information relates or obtaining their consent, any information in respect of a clinical trial that has ceased to be confidential business information.”
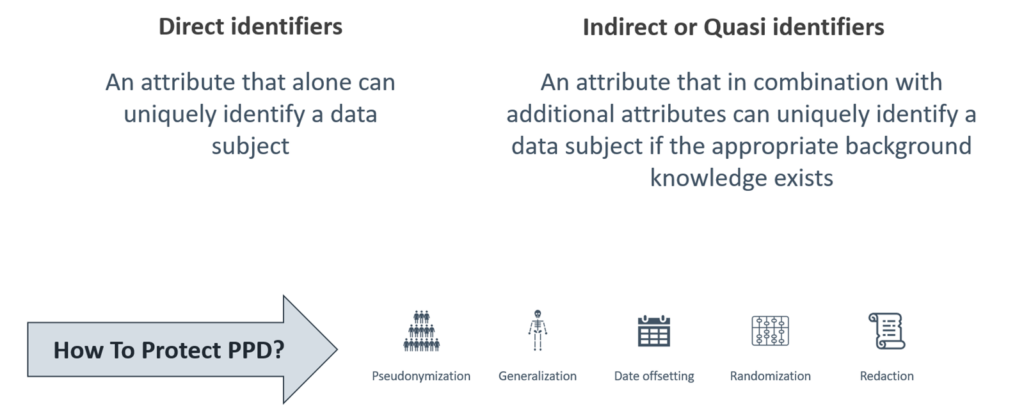
Figure 1. PPD can be protected via redaction or anonymization methods.
Key Factors
Health Canada has consistently rejected fully redacted sections (including case narratives, patient-level tables, listings, and figures). Some sponsors have received a notice with their published drug submission on the Health Canada PRCI portal stating their non-conformance to the guidance when sections are fully redacted. Consider an approach that maximizes data utility while protecting patient privacy and conforming to the Health Canada guidance. Certara employs a targeted redaction/anonymization strategy that maximizes data utility while maintaining a low risk of re-identification of trial participants.
Confidential Business Information (CBI)
When preparing clinical documents for publication, consider what company information qualifies as CBI (known as Commercially Confidential Information (CCI) by the European Medicines Agency). Health Canada has strict criteria: information must be non-public and cause economic harm if disclosed. They rarely accept CBI claims, so carefully evaluate before applying.

Remember, the Food and Drugs Act empowers regulators to disclose any non-confidential information. Sections C.08.009.3 and 43.13s of relevant regulations explicitly state they can publish unredacted CBI without notifying sponsors, assuming the documents lack it. Minimize CBI claims and strictly follow Health Canada’s guidelines to avoid potential disclosure of sensitive information.
Personal Information
If a Sponsor doesn’t hide CBI in their documents, there may be none to hide. When it comes to PI, it’s a different story. Even a Phase 1 trial without any reported issues or detailed stories contains basic details about participants like their gender, age, race, height, weight, or BMI.
When submitting to Health Canada’s PRCI Portal, sponsors must comply with The Privacy Act and the Personal Information Protection and Electronic Documents Act (PIPEDA):
- Privacy Act, Protection of Personal Information, Disclosure of Personal Information: Personal information under the control of a government institution shall not, without the consent of the individual to whom it relates, be disclosed by the institution.
- PIPEDA 4.5, Principle 5 – Limiting Use, Disclosure, and Retention: Personal information shall not be used or disclosed for purposes other than those for which it was collected, except with the consent of the individual or as required by law.
Sponsors must comply with the PRCI guidance, so Health Canada doesn’t release participants’ information without consent.
Infrastructure and Partnerships
Thoughtful evaluation of your company’s ability to meet the transparency requirements and proactive preparation of the clinical submission documents are the foundation for a successful Health Canada PRCI-compliant submission. Important questions to ask include:
- What is our transparency strategy?
- Who should be part of our transparency team?
- How will our company meet the transparency requirements for a successful Health Canada PRCI submission?
What are the Penalties?
While Health Canada expects compliance, what happens if a sponsor resists? Organizations conducting business in Canada are subject to PIPEDA. Penalties aren’t explicitly mentioned in the PRCI guidance document, but violating privacy laws like PIPEDA carries hefty fines.
- The Office of the Privacy Commissioner enforces PIPEDA, sets national privacy standards, and requires breach reporting. Businesses can be fined up to CAD $100,000 per violation.
- Breaches can lead to lawsuits from affected individuals.
Similar regulations exist in other regions:
- U.S.: Health Insurance Portability and Accountability Act of 1996 (HIPAA)
- Europe: EU General Data Protection Regulation (GDPR)
Understanding your legal and ethical responsibilities when submitting clinical trial documents to regulators is good business. Certara can prepare anonymized clinical trial document packages for publishing on Health Canada’s PRCI Portal. Learn more about our transparency and disclosure services here.
References
- Department of Justice Canada. Justice at a Glance: Personal Information Protection and Electronic Documents Act (PIPEDA). Accessed January 30, 2024.
- Canada Gazette. Regulations Amending the Food and Drug Regulations (Vanessa’s Law) — Regulatory Impact Analysis Statement. Canada Gazette. December 9, 2017. Accessed January 30, 2024.
- Health Canada. Profile: Public Release of Clinical Information (PRCI) Guidance. Published March 2019. Accessed January 30, 2024.
- Office of the Privacy Commissioner of Canada. Personal Information Protection and Electronic Documents Act (PIPEDA). Accessed January 30, 2024.
This blog was originally published by April 26, 2021, by Holly Curry and Shawn Lantz and has since been updated by Lisa Mantifel on February 9, 2024.



