October 16, 2025
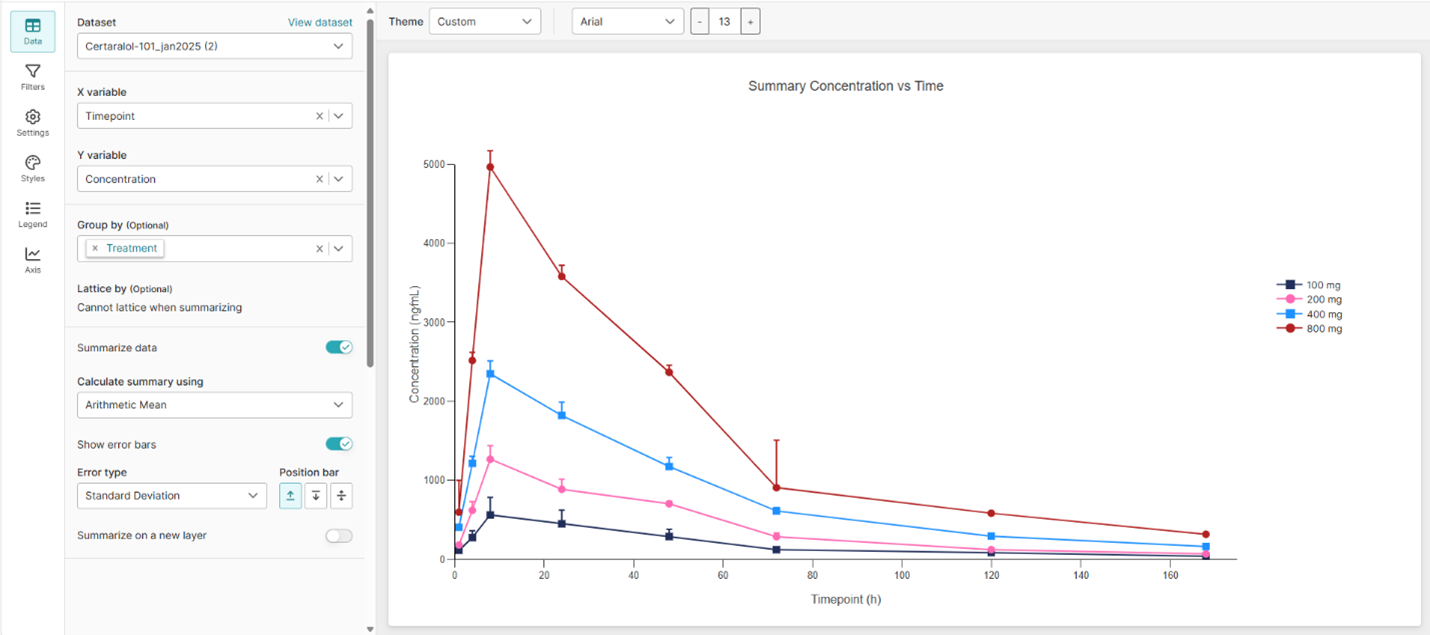

Sneak Preview: Introducing TFL Studio
Watch our on-demand webinar for a first look at TFL Studio in action.
Learn more about TFL Studio
The “Easy Button” for Your Everyday PK/PD Analysis


Principal Research Scientist
Dr. Kristin Follman is a Principal Research Scientist at Certara, where she is a member of the team building the TFL module. She obtained a PhD in Pharmaceutical Sciences from the University at Buffalo where her work focused on drug transporters in the treatment of overdose and renal impairment. Prior to joining the software division, Kristin worked as a consultant at Certara for 5 years where her area of expertise was quantitative clinical pharmacology, with a focus on translational PK/PD modeling and simulation.
FAQS
What are TLFs (Tables, Listings, and Figures) in clinical trials?
TLFs, or Tables, Listings, and Figures (sometimes called TFLs), are standardized formats used in clinical and PK/PD reporting to present study data clearly and reproducibly. They help regulatory reviewers assess drug safety and efficacy by summarizing key data like patient demographics, lab results, and dose–response relationships. Each element serves a purpose: tables for summary data, listings for individual records, and figures for visual insights.
How does TLF automation improve PK/PD reporting efficiency?
Automating TLF generation eliminates repetitive manual steps, enabling faster, more consistent reporting. With tools like TFL Studio, analysts can generate publication-ready outputs from templates without coding, ensuring traceability and regulatory compliance. Automation also supports reproducibility across studies and reduces quality control cycles.
What are the main differences between creating TLFs in Excel and TFL Studio?
Excel offers flexibility but lacks audit trails and reproducibility, making it risky for regulated studies. TFL Studio, by contrast, provides an audit-compliant, template-driven workflow where TLFs can be generated automatically and reviewed collaboratively. It combines the ease of Excel’s interface with the traceability of validated software, streamlining compliance and efficiency.
How does TFL Studio ensure compliance with FDA and EMA standards?
TFL Studio was designed with regulatory compliance in mind, aligning with ICH E3 and 21 CFR Part 11 standards. It maintains audit logs, version control, and secure data integration through Phoenix Integral. These features ensure all generated outputs are traceable, reproducible, and ready for regulatory submission.
Be the first to experience the new Phoenix TFL Studio





