February 3, 2025
- Feb
- Mar
- Apr
- May
- Jun
- Jul
- Aug
Figure 1: Example of a “Standard” NDA Timeline
- Feb
- Mar
- Apr
- May
- Jun
- Jul
- Aug
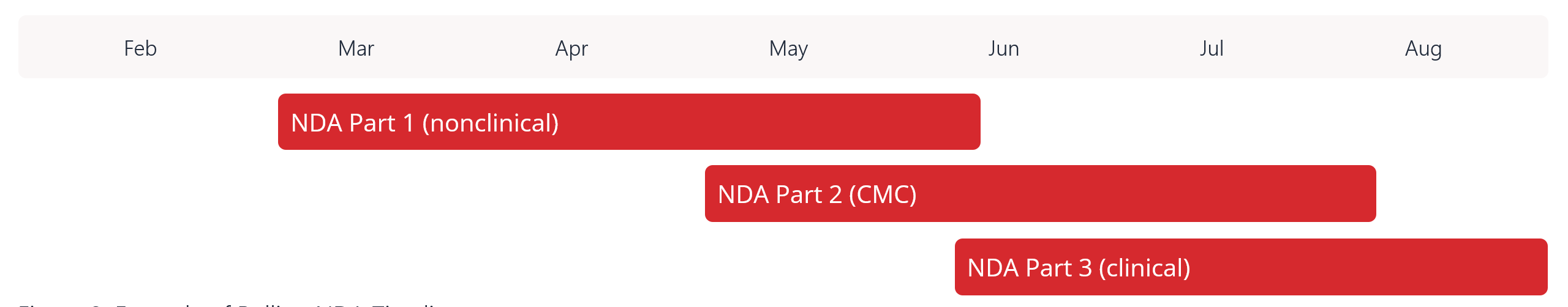
Figure 2: Example of Rolling NDA Timeline
Although it may seem overwhelming to approach a rolling submission, this approach is becoming more popular and is worth considering if you want to accelerate your timeline to approval. For insights on other regulatory trends and managing eCTD submissions, watch On-Demand Webinars on Regulatory topics on Certara’s Resources page, including:

Author’s note: this blog post was originally published in April 2021 and has been updated for accuracy and comprehensiveness.

Associate Principal, Regulatory Operations Specialist
Janet Shoshitaishvili is an experienced Regulatory Operations professional who has been assisting Certara clients for over 5 years with regulatory submission publishing for investigational and marketing applications. Her background includes over 25 years working in the pharmaceutical industry, focused on various aspects of regulatory submissions and compliance.
Contact us