The Challenge of Time-Consuming Bioequivalence studies
The pharmaceutical landscape is witnessing a significant transformation in how bioequivalence (BE) studies are conducted, particularly for complex and locally acting generics. Bioequivalence is a critical concept in the development of generic drugs, ensuring that two formulations of a drug reach systemic circulation at equivalent rates and extents. The traditional methods of assessing bioequivalence often involve extensive clinical trials, which can be time-consuming and costly. However, the regulatory acceptance of Physiologically-Based Pharmacokinetic (PBPK) modeling is changing the game, offering innovative solutions that streamline the approval process for generic drugs, reducing or eliminating BE trials.
PBPK Modeling: A Mechanistic Approach
For locally acting generics—those that exert their effects at specific sites like the skin or lungs—PBPK modeling provides a detailed, mechanistic framework. This approach integrates drug characteristics, product quality attributes, and physiological factors relevant to the site of action. By doing so, it enhances our understanding of how product quality influences efficacy, thereby informing regulatory guidance and mitigating risks associated with formulation changes.
A notable example of PBPK modeling in action is the FDA’s approval of a generic diclofenac sodium topical gel (1%). In this case, PBPK modeling established a clear link between formulation properties and local bioavailability, paving the way for a more streamlined BE pathway. Utilizing the Simcyp Simulator’s MPML-MechDermA model, this approval set a new standard for regulatory processes concerning complex generics.
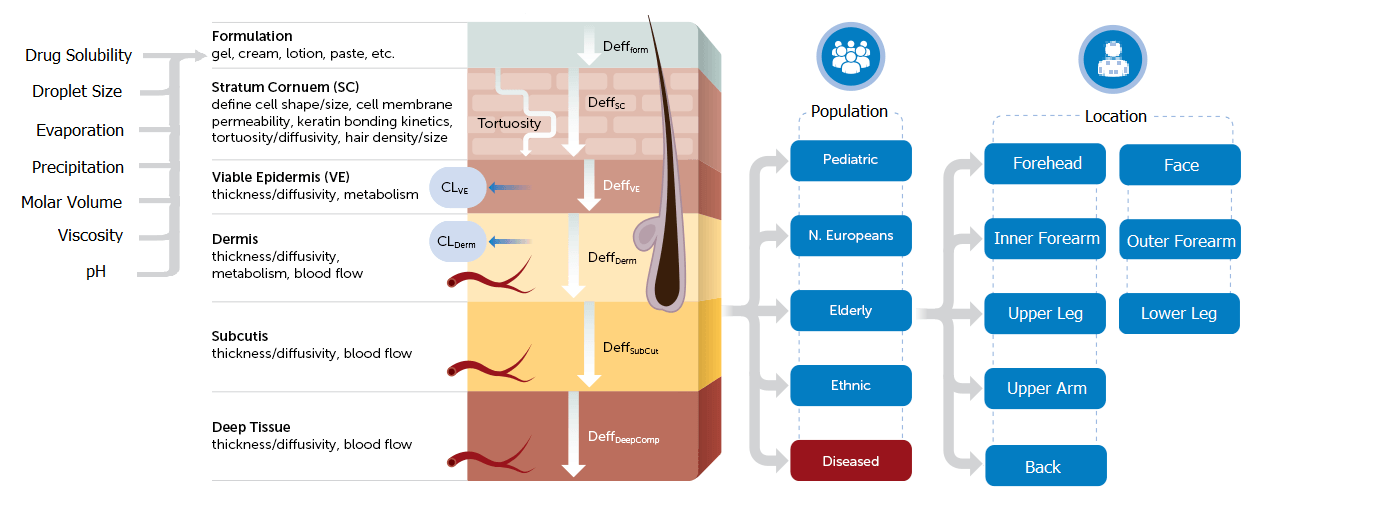
Figure 1. Simcyp’ s Multi-Phase Multi-Layer (MPML) MechDermA Model
Expanding the Horizons of Virtual Bioequivalence (VBE)
Recent advancements in PBPK modeling have broadened its applicability across various formulations, including:
- Oral Products: Tablets, capsules, and suspensions can leverage VBE capabilities, which include support for dissolution specifications and BCS-based biowaivers beyond Class I and III compounds.
- Non-Oral Routes: Models from Simcyp facilitate VBE for non-oral routes such as intramuscular, intravenous, and vaginal applications, expanding regulatory submission options and minimizing clinical trial needs.
- Topical Formulations: The success of the diclofenac gel serves as a model for complex generics, establishing VBE as a viable regulatory pathway for similar products e.g. LAIs.
These advancements not only save time and resources but also accelerate access to affordable generics, ultimately benefiting patients and healthcare systems alike.
Key Takeaways for PBPK Modeling in Bioequivalence
- Real-World Impact: PBPK modeling has successfully supported multiple VBE approvals including the approval of a generic diclofenac sodium gel, validating VBE for complex topical formulations.
- Connecting Product Attributes with BE: Mechanistic models effectively link formulation properties with bioavailability, supporting BE for both oral and locally acting products.
- Growing Regulatory Acceptance: The acceptance of VBE continues to grow, facilitating the development and approval of generic drugs.
- Reduction of Clinical Trials: PBPK modeling can often replace or reduce the need for clinical trials, expediting the approval process and lowering costs. This is particularly beneficial for drugs where traditional BE studies are complex or ethically challenging.
- Reduced Costs and Time: PBPK modeling can significantly reduce or eliminate the need for expensive and time-consuming clinical trials.
- Risk Assessment for Drug-Drug Interactions (DDIs): PBPK models are instrumental in assessing potential drug-drug interactions early in the development process, which is crucial for regulatory approval.
Looking Ahead: The Future of PBPK Modeling and VBE
The bioequivalence landscape is rapidly evolving, with PBPK modeling and VBE leading the charge. This innovative approach promises to expand biowaiver applications, reduce time-to-market, and lower costs associated with generic drug development.
Connect with us to explore how PBPK modeling can enhance your bioequivalence strategies and streamline regulatory submissions.
The future of generic drug development is bright, and PBPK modeling is at the forefront of this exciting transformation.
Access Our Latest White Paper: Overcome Research Hurdles in Generic Drug Development
Are costly bioequivalence studies holding back your generic drug pipeline? Don’t let traditional methods slow you down! Read our latest white paper to discover innovative solutions for navigating the complexities of generic drug development.
This blog was originally published on November 1, 2022 by Nikunjkumar Patel. It was updated on November 12, 2024 for accuracy and comprehensiveness.



