A new class of complex biologics has emerged in recent years. These are classified in a broad category of live biotherapeutic products, and regulatory guidances have been written across the spectrum of such new interventions. Unlike traditional new therapies, these products do not hit the pharmacological target nor engage with the target in any meaningful way like a small molecule drug. Instead, such products interact with the microbiome and exert their effects through the set of host-microbiota interactions. Okay I introduced a new concept of microbiome.
What’s a microbiome?
The microbiome is an ecosystem that exists in our body that consists of all microbes (e.g., bacteria, fungi, etc.), that have a role to play in health of the human being. The latter implies interaction with the host. Perturbations in the microorganisms can occur so that the normally benign microbes are displaced by pathogenic microbes that can cause a variety of diseases. Now, just imagine a pharmaceutical product that consists of such live microorganisms that are given to treat these diseases.
The US FDA defines the LBP as a biologic that “1) contains live organisms, such as bacteria; 2) is applicable to the prevention, treatment, or cure of a disease or condition of human beings; and 3) is not a vaccine.” The guidance goes on and makes further specifications and clarifications particularly that these are “not filterable viruses, oncolytic bacteria, or products intended as gene therapy agents and, as a general matter, are not administered by injection.”
The guidance also gives a couple of examples of LBPs such as one or more strains of lactobacilli administered orally to treat patients with ulcerative colitis or administered vaginally to prevent bacterial vaginosis. A cursory scan of clinicaltrials.gov reveals at least twenty clinical trials with an LBP currently recruiting or inactive. That is not much, and part of the reason such products are not that popular relates to manufacturing issues.
The recent approval of REBYOTA, live fecal microbiota, indicated for preventing recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older, following antibiotic treatment for recurrent CDI may peak interest in such LBPs.
Here are the three key considerations for developing LBPs:
Choice of the strain
This is a key pharmacology question. Ideally, the LBPs consists of a single strain or active substance. When developers consider a multi-strain product, then they need to characterize the biological properties (and mechanism of action) of each strain added to the LBP formulation. Rationally, a single strain LBP is much easier to develop and characterize than a multi-strain product. In fact, Carlson and co-workers outline specific considerations for strain characterization due to safety issues including, but not limited to, being able to identify potentially unfavorable effects of all strains included in the product. They also suggest that such strain characterization include information on virulence factors and antibiotic resistance genes. As one can imagine, risk increases exponentially as the number of strains in the product increases.
Dose selection and proof of biology
There are no unique approaches to dose selection beyond existing guidance on safe starting doses for biologics. However, the choice of the dose or dose dynamic window depends on the target site of action, accessibility of the microbe to the site of action, amount of LBP needed for coverage as well as biodistribution and clearance of the product. Because of the nature of the LBP, the selection of the highest dose studied in clinical trials must be that at which risk of any translocation to normally sterile tissues and infection is contained and/or monitored. A pre-IND meeting is a helpful forum for interacting with regulatory agency experts as it relates to strategy for dose selection and choice of endpoints.
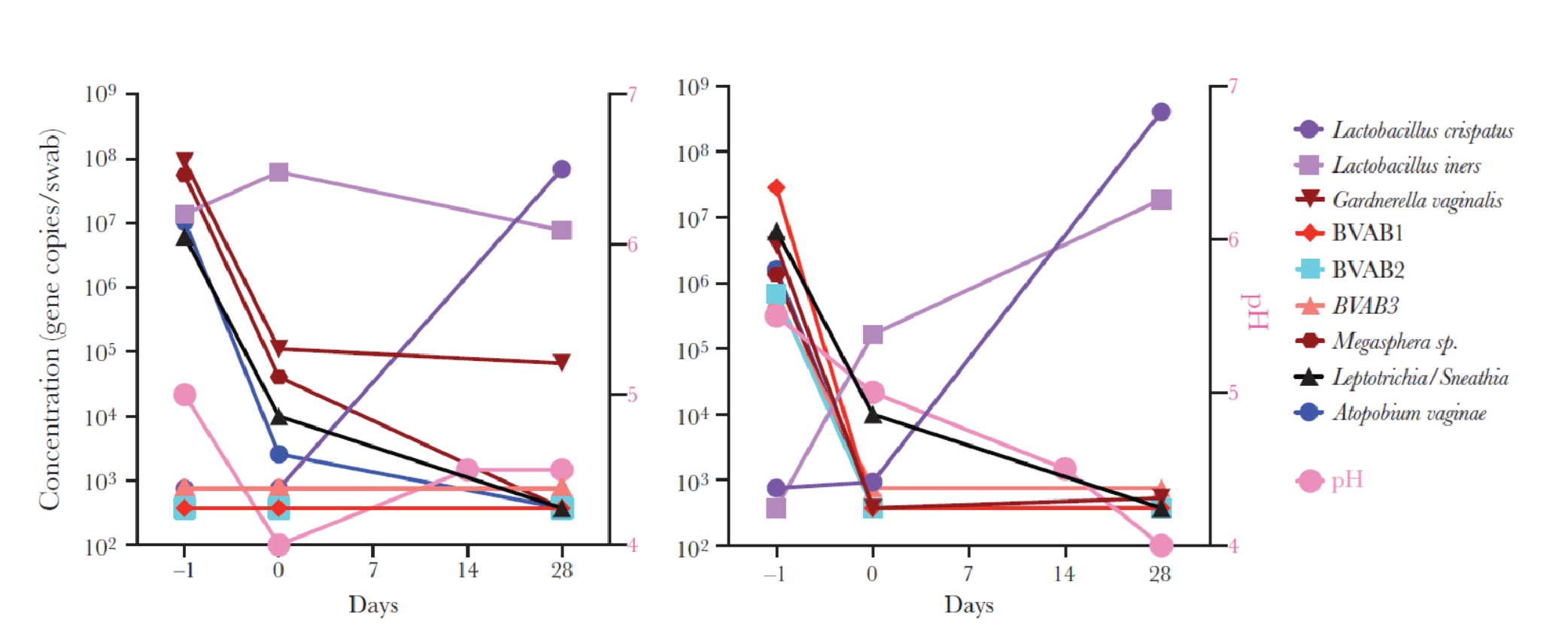
Much can be learned from the clinical trial experience of a vaginal microbiome-based LBP, Lactin-V. There is a known association between depletion of vaginal Lactobacillus (notably L. crispatus), with bacterial vaginosis (BV) and certain urinary tract infections. Lactin-V is a single strain of L. crispatus under development for the prevention of BV.
Lactin-V has undergone advanced clinical testing as it relates to activity and safety. In a proof-of-concept trial where Lactin-V was administered by an applicator (NCT00635622), BV participants received metronidazole vaginal gel followed by LACTIN-V (2 × 109 CFU/applicator) or matching placebo applicator daily for 5 days, then once weekly for 2 weeks. Results indicated that 61% in the LACTIN- V group were colonized with the active substance (Figure 2). This study was instrumental in understanding how vaginal colonization with L. crispatus following LACTIN-V treatment was associated with reduced levels of BV-associated anaerobes. Such an approach could potentially reduce the risk of BV recurrence.
Drug interaction assessment
It is well known that bacteria in the gut can metabolize orally administered drugs. It is conceivable that LBP products under development should consider the drug interaction risks of concomitant medications. The effect of LBPs on such concomitant medications should be studied, experimentally or in-silico. Tools such as physiologically-based pharmacokinetic (PBPK) modeling can identify potential signals that can aid in such an assessment.
If you are developing an LBP and need help with your clinical development plan, our clinical pharmacologists can help you. Learn more about our consultants here.
References for further reading
- Dreher-Lesnick SM, Stibitz S, Carlson PE Jr. U.S. Regulatory Considerations for Development of Live Biotherapeutic Products as Drugs. Microbiol Spectr. 2017 Oct;5(5). doi: 10.1128/microbiolspec.BAD-0017-2017.
- FDA. Early Clinical Trials With Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information (2016).
- Hemmerling A, Harrison W, Schroeder A, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex Transm Dis 2010; 37:745–50.
- Lagenaur LA, Hemmerling A, Chiu C, Miller S, Lee PP, Cohen CR, Parks TP. J Connecting the Dots: Translating the Vaginal Microbiome Into a Drug. Infect Dis. 2021 Jun 16;223(12 Suppl 2):S296-S306. doi: 10.1093/infdis/jiaa676.



