December 5, 2024

Using Clinical Pharmacology & Pharmacometrics to Accelerate Cell Therapy Development: A Patient’s CAR-T Journey
To learn more about best practices for cell therapeutics development, please watch this webinar.
References
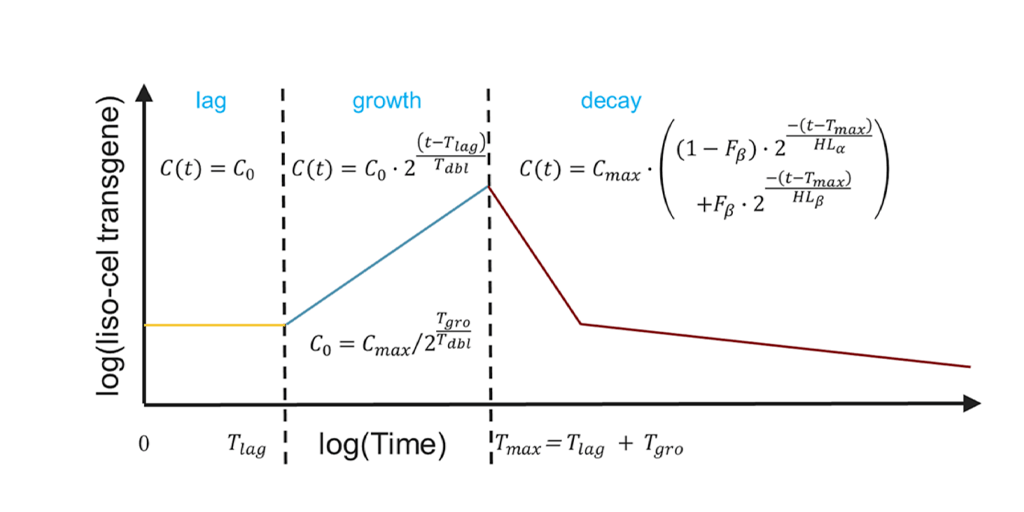
Ogasawara K, Dodds M, et al. Population Cellular Kinetics of Lisocabtagene Maraleucel, an Autologous CD19‑Directed Chimeric Antigen Receptor T‑Cell Product, in Patients with Relapsed/Refractory Large B‑Cell Lymphoma. Clinical Pharmacokinetics. 2021 Dec;60(12):1621-1633. doi: 10.1007/s40262-021-01039-5. Epub 2021 Jun 14.
FDA draft guidance, March 2022: Considerations for the development of CAR-T cell products
This blog was originally published on September 9, 2022 and has since been updated.

Senior Transparency Specialist
Rajesh is a scientific key opinion leader with 25+ years in drug development, specializing in model-informed strategies for biologics, vaccines, and small molecules. Currently a Distinguished Scientist at Certara, he leads strategic consulting and the CDDS centers of excellence. Previously, he founded Merck’s quantitative clinical pharmacology department and held key roles at Aventis and Bristol-Myers Squibb. Rajesh holds a PhD in Pharmaceutical Sciences (University of British Columbia) and an MBA in Strategy and Innovation (Warwick). Consistently recognized among the top 2% of influential scientists, his work includes 100+ publications, 89 posters, and 4 books. He is an elected fellow of AAPS.

Vice President
Kathryn is a clinical pharmacology consultant and Team Lead within the Integrated Drug Development group at Certara. In addition, she leads the Complex Biologics Integrated Practice Area that specialises in providing clients with expert support in areas such as cell therapies, RNA technologies, gene therapies, fusion proteins, ADCs, bispecific antibodies, etc. Prior to joining Certara in 2020, Kathryn worked in clinical pharmacology & pharmacometrics in the pharmaceutical industry for more than 20 years, working across all phases of development.