In a long-awaited development, Italy’s Medicines Agency, AIFA, underwent transformative changes on January 30th, 2024. Following the appointment of President Giovanni Nisticò, these have become fully operational.
This has been a recurrent topic of discussion, with talks of merging the Scientific Technical Advisory Committee (CTS) and the Price and Reimbursement Committee (CPR). However, the COVID-19 pandemic delayed these plans, leading to a continuous extension of the current committee members’ tenure.
The road to transforming AIFA into a more streamlined and efficient regulatory and health technology assessment (HTA) body accelerated with the State-Regions Conference agreement on November 15, 2023. Sources from the Ministry of Health (MoH) confirmed that the AIFA CTS and CPR were likely to remain operational for a maximum of 45 days after the 1st of Dec 2023 for a transitional phase.
The CTS and the CPR have continued their work, respectively on technical assessments and pricing and reimbursement negotiations, until mid-January. This extension aligned with the MoH’s assertion that all final steps, including appointments of the AIFA president, scientific and administrative directors, and CSE members, would be completed by mid-January 2024.
So, what can we expect from the new AIFA?
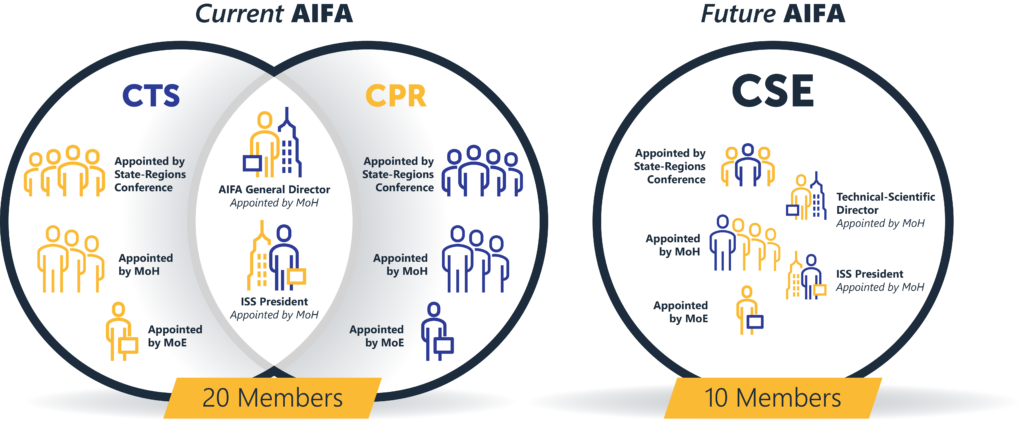
- Elimination of General Director
The General Director position no longer exists, leaving only the President, who becomes the legal representative of the agency.
- Administrative and Scientific-Technical Directors
The President will be supported by an Administrative Director and a Scientific Technical Director.
- Single Commission for Regulatory Activity
A single commission, the Scientific and Economic Commission for Medicines (CSE), consisting of 10 members, will replace the current 20-member strong CPR and CTS. This commission will handle regulatory activities such as approving and determining drug prices.
- Composition of the Scientific and Economic Commission
The commission will comprise of 10 members:
- The Technical-Scientific Director of the agency.
- The President of the Higher Institute of Health (ISS) or a delegate.
- One technician appointed by the Ministry of Economy.
- Three members appointed by the State-Regions Conference.
- Four members designated by the MoH with each having at least 5 years of experience in drug evaluation, pricing, and pharmacoeconomics.
The Technical-Scientific Director of the agency.
- Appointing System
The president will be chosen by the ministry in agreement with the Regions, providing Regions with a binding opinion. The appointment of administrative and scientific directors will not have a veto from regions but will involve their consultation.
We are now a few months into the new AIFA. The priorities and strategies of the reformed body have been widely communicated and are becoming increasingly evident.
Enhanced stakeholders’ communication
AIFA is enhancing its dialogue with relevant stakeholders. Article 11 of its new regulation recognizes scientific societies and patient associations for consultative purposes. This is a first in Italy, where patients’ associations were effectively cut out from the decision-making progress at the institutional level.
Manufacturers have also been included in this enhanced dialogue. In May, a technical table between Farmindustria and AIFA was organized to reduce access times for drugs. The main goal was to identify tools that can simplify and prioritize innovative drug approvals. Among them, a new online platform, announced by the Technical-Scientific Director Pierluigi Russo, aims at a more transparent communication regarding the CSE procedure and the updating of the reimbursement dossier guidelines.
Less bureaucracy
AIFA wants to streamline and simplify the current processes. In July, it convened scientific societies and GPs’ organizations to a Technical Table for the revision of the AIFA notes and therapeutic plans.
Therapeutic plans define the conditions of use and reimbursement to have best practices applied across the nation. AIFA notes can be seen as reimbursement restrictions for the approved indications. These plans currently involve a quarter of all authorized and reimbursed drugs.
Access to drugs under therapeutic plans incurs extra bureaucracy, administrative hurdles for patients treated outside their region of residence, and the recurrent renewal of the plans (every 6-12 months). All these prompt delayed initiations and access complications. This is why AIFA wants to streamline and foster the more balanced use of such tools.
Faster access to medications
The AIFA president has also suggested a new early access reimbursement model for all new drugs or advanced therapies right after the EMA approval. An initial price would be calculated by AIFA’s algorithm and applied automatically. This way, access would be granted without waiting for pricing negotiation outcomes.
After negotiations take place, a potential price adjustment would be applied to ensure a sustainable and ethical approach. This new access model appears to have elements of the Japanese pricing algorithm and the German free pricing period. However, some have already associated it with the French early access program. Italy is already well-equipped with early access programs. So, it will be interesting to monitor how this idea will materialize and develop.
What can we expect from the new AIFA?
AIFA’s committee changes are unlikely to significantly impact their current pricing and reimbursement methodology. However, we can reasonably hypothesize differences in assessment timelines.
The new CSE will only have half of the staff that the previous CTS and CPR committees had. At the same time, the current back-and-forth dynamic that happened between the two commissions could be avoided thanks to a more streamlined approach introduced by the unique CSE working group.
The Italian agency stands at a pivotal juncture. It can evolve into a more agile AIFA, harmonizing the technical and economic facets of the CSE to assess a drug’s value holistically and set its price accordingly. Or it may adopt a slower pace constrained by staff headcounts. Then it could be influenced more by CSE’s economic side with factors like budgetary considerations ultimately influencing the drug’s benefit assessment.
Regardless, AIFA’s new objectives are ambitious. If achieved, they could make launching drugs in Italy more attractive thanks to enhanced stakeholder communication, reduced bureaucracy, and a new way of gaining faster market access.
If you need help with your drug’s Italian market access strategy, check out this webpage to learn more about how our experts can help!
This blog was originally published on February 5, 2024, and was updated on Oct 2, 2024.



