July 23, 2025
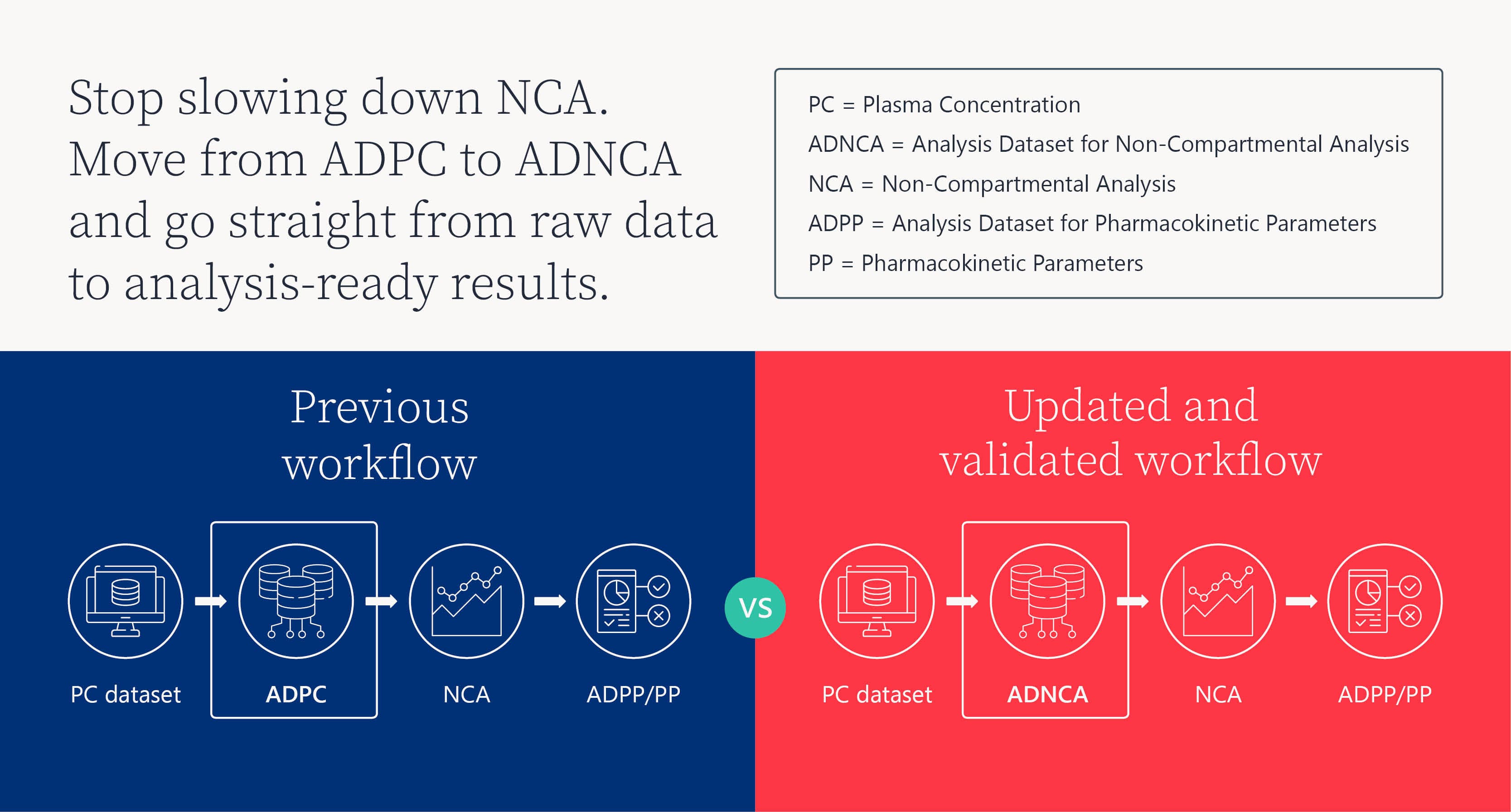
"ADNCA can be considered that standardized bridge between raw data and analyzed outputs, making NCA reproducible and ready for regulatory submission”
Alexia Di Quinzio, Pharmacokinetic Scientist
"By submitting ADNCA, you’ll get a faster review, happier reviewers, fewer IRs, and ultimately—more time on patent."
Jeffrey Abolafia, Director of Product Innovation, P21
"You can drop ADNCA right into Phoenix without doing any complex data transformations… no manual entry needed."
Alexia Di Quinzio
"ADNCA variables don’t just tell us what to analyze—they define how we analyze it."
Alexia Di Quinzio
"It’s not obligatory—yet—but reviewers are starting to like it, expect it, and it fits well in their toolbox."
Jeffrey Abolafia
"NCA remains the gold standard for early-phase PK. ADNCA makes it faster, clearer, and ready for what’s next."
Alexia Di Quinzio

Want to learn more about ADNCA?
Watch our on-demand webinar to hear how top PK scientists are using ADNCA to modernize NCA workflows, reduce risk, and speed up submissions. Or connect with our experts to explore how ADNCA fits into your drug development program.

Marketing Director, Quantitative Science Services
With over 22 years of experience in hospitals, health systems, associations, life sciences, physician practices, and suppliers, Erika is an experienced marketing strategist and supports the Quantitative Science Services offering with Go-to market planning and execution.